General properties of p elements. P-elements. Carbon and its compounds
Elements in Mendeleev's periodic system are divided into s-, p-, d-elements. This subdivision is carried out on the basis of how many levels the electron shell of an atom of an element has and at what level the filling of the shell with electrons ends.
To s-elements refer elements IA-groups - alkali metals. Electronic formula of the valence shell of alkali metal atoms ns1. The stable oxidation state is +1. Elements IA groups have similar properties due to the similar structure of the electron shell. With an increase in the radius in the Li-Fr group, the bond of the valence electron with the nucleus weakens and the ionization energy decreases. Atoms of alkaline elements easily donate their valence electron, which characterizes them as strong reducing agents.
Restorative properties are enhanced with increasing serial number.
To p-elements include 30 items IIIA-VIIIA-groups periodic system; p-elements are located in the second and third small periods, as well as in the fourth to sixth large periods. Elements IIIA-groups have one electron in the p orbital. AT IVA-VIIIA-groups the filling of the p-sublevel up to 6 electrons is observed. General electronic formula of p-elements ns2np6. In periods with an increase in the nuclear charge, the atomic radii and ionic radii of p-elements decrease, the ionization energy and electron affinity increase, electronegativity increases, the oxidative activity of compounds and the non-metallic properties of elements increase. In groups, the radii of atoms increase. From 2p elements to 6p elements, the ionization energy decreases. The metallic properties of the p-element in the group increase with increasing serial number.
To d-elements includes 32 elements of the periodic system IV–VII big periods. AT IIIB-group the atoms have the first electron in the d-orbital, in subsequent B-groups the d-sublevel is filled up to 10 electrons. General formula of the outer electron shell (n-1)dansb, where a=1?10, b=1?2. With an increase in the ordinal N, the properties of the d-elements change slightly. For d-elements, the atomic radius slowly increases, and they also have a variable valence associated with the incompleteness of the pre-external d-electron sublevel. In the lower oxidation states, d-elements show metallic. St. Islands, with an increase in the order. N in groups B they decrease. In solutions, d-elements with the highest degree of oxidation show acidic and oxidizing properties, and vice versa at lower degrees of oxidation. Elements with int. step. oxidation show amphoteric. St. Islands.
covalent bond.
The chemical bond carried out by common electron pairs arising in the shells of the bound atoms having antiparallel spins is called atomic or covalent bond. Covalent bond is two-electron and two-center (holds nuclei). An atom at its outer energy level can contain from one to eight electrons. Valence electrons are the electrons of the outer, outer electron layers involved in chemical bonding. Valence- the property of the atoms of an element to form a chemical bond.
p-elements are:
- in the 1st period - no p-elements
- in the 2nd period - -
- in the 3rd period - -
- in the 4th period - -
- in the 5th period - -
- in the 6th period - -
P-elements include non-transition metals and most non-metals. P-elements have different properties, both physical and mechanical. P-non-metals are highly reactive, as a rule, substances with a strong electronegativity, P-metals are moderately active metals, and their activity increases towards the bottom of the PSCE.
see also
- -elements
- -elements
- -elements
- -elements
Wikimedia Foundation. 2010 .
See what "P-elements (chemical)" are in other dictionaries:
- (a. chemical elements; n. chemische Elemente; f. elements chimiques; i. elementos quimicos) components of simple and complex bodies, which are a collection of atoms with the same charge of atomic nuclei and the same number of electrons in ... Geological Encyclopedia
Chemical elements- elements of Mendeleev's Periodic Table of Elements, in which each element is the whole set of atoms with the same charge of atomic nuclei and the same number of electrons in the atomic shell. Currently, the number of known elements is 118... Beginnings of modern natural science
The simplest form of matter that can be identified by chemical methods. These are the constituent parts of simple and complex substances, which are a collection of atoms with the same nuclear charge. The charge of the nucleus of an atom is determined by the number of protons in... Collier Encyclopedia
Periodic system of chemical elements of D. I. Mendeleev H ... Wikipedia
Each E. x. this is a collection of atoms with the same charge of atomic nuclei and the same number of electrons in the atomic shell. The atomic nucleus consists of protons, the number of which is equal to the atomic number (See Atomic number) of the element, and neutrons, ... ... Great Soviet Encyclopedia
Sets of atoms with a certain nuclear charge Z. D. I. Mendeleev determined E. x. so: the material parts of simple or complex bodies, to rye give them a known set of physical. and chem. St. v. Interrelations E. x. reflects the periodic system ... ... Chemical Encyclopedia
Chemical elements- components of the whole variety of simple and complex substances. Each chemical element is a collection of atoms with the same charge of atomic nuclei and the same number of electrons in the atomic shell. The atomic nucleus is made up of... Encyclopedic Dictionary of Metallurgy
The enormous variety of phenomena and substances of nature, in its study, human thought has always sought to simplify by assuming, if not the complete unity of the basic E. (Democritus, Epicurus), then at least with the help of a small number of E., ... ... Encyclopedic Dictionary F.A. Brockhaus and I.A. Efron
- (abbr. HIT) devices in which the energy of chemical reactions occurring in them is directly converted into electrical energy. Contents 1 History of creation 2 Principle of operation ... Wikipedia
Books
- Chemical elements, Vaitkene Lyubov Dmitrievna. Fe, Au, Cu Ferrum, aurum, cuprum You don't know what these words mean yet, but would you like to know? Then this book is your faithful assistant in mastering such a difficult science as chemistry. After reading…
The p-elements of the periodic system include elements with a valence p-sublevel. These elements are located in III, IV, V, VI, VII, VIII groups, main subgroups. In a period, the orbital radii of atoms decrease with increasing atomic number, but generally increase. In subgroups of elements, as the element number increases, the sizes of atoms generally increase rather than decrease. p-elements of group III Group III p-elements include gallium Ga, indium In and thallium Tl. By the nature of these elements, boron is a typical non-metal, the rest are metals. Within the subgroup, a sharp transition from non-metal to metals can be traced. The properties and behavior of boron are similar, which is the result of the diagonal affinity of elements in the periodic system, according to which a shift in the period to the right causes an increase in the non-metallic character, and down the group - a metallic one, therefore elements similar in properties turn out to be located diagonally side by side, for example Li and Mg, Ber and Al, B and Si.
The electronic structure of the valence sublevels of Group III p-element atoms in the ground state has the form ns 2 np 1 . In compounds, boron and trivalent, gallium and indium, in addition, can form compounds with +1, and for thallium the latter is quite characteristic.
p-Elements of group VIII Group VIII p-elements include helium He, neon Ne, argon Ar, krypton Kr, xenon Xe and radon Rh, which constitute the main subgroup. The atoms of these elements have complete outer electron layers, so the electronic configuration of the valence sublevels of their atoms in the ground state has the form 1s 2 (He) and ns 2 np 6 (other elements). Due to the very high stability of electronic configurations, they are generally characterized by high ionization energies and chemical inertness, which is why they are called noble (inert) gases. In the free state, they exist in the form of atoms (monatomic molecules). Helium (1s 2), neon (2s 2 2p 6) and argon (3s 2 3p 6) atoms have a particularly stable electronic structure, so valence-type compounds are unknown to them.
Krypton (4s 2 4p 6), xenon (5s 2 5p 6) and radon (6s 2 6p 6) differ from the previous noble gases in larger atomic sizes and, accordingly, lower ionization energies. They are able to form compounds that often have low resistance.
Elements in Mendeleev's periodic system are divided into s-, p-, d-elements. This subdivision is carried out on the basis of how many levels the electron shell of an atom of an element has and at what level the filling of the shell with electrons ends.
To s-elements refer elements IA-groups - alkali metals. Electronic formula of the valence shell of alkali metal atoms ns1. The stable oxidation state is +1. Elements IA groups have similar properties due to the similar structure of the electron shell. With an increase in the radius in the Li-Fr group, the bond of the valence electron with the nucleus weakens and the ionization energy decreases. Atoms of alkaline elements easily donate their valence electron, which characterizes them as strong reducing agents.
Restorative properties are enhanced with increasing serial number.
To p-elements include 30 items IIIA-VIIIA-groups periodic system; p-elements are located in the second and third small periods, as well as in the fourth to sixth large periods. Elements IIIA-groups have one electron in the p orbital. AT IVA-VIIIA-groups the filling of the p-sublevel up to 6 electrons is observed. General electronic formula of p-elements ns2np6. In periods with an increase in the nuclear charge, the atomic radii and ionic radii of p-elements decrease, the ionization energy and electron affinity increase, electronegativity increases, the oxidative activity of compounds and the non-metallic properties of elements increase. In groups, the radii of atoms increase. From 2p elements to 6p elements, the ionization energy decreases. The metallic properties of the p-element in the group increase with increasing serial number.
To d-elements includes 32 elements of the periodic system IV–VII big periods. AT IIIB-group the atoms have the first electron in the d-orbital, in subsequent B-groups the d-sublevel is filled up to 10 electrons. General formula of the outer electron shell (n-1)dansb, where a=1?10, b=1?2. With an increase in the serial number, the properties of d-elements change insignificantly. For d-elements, the atomic radius slowly increases, and they also have a variable valence associated with the incompleteness of the pre-external d-electron sublevel. In the lower oxidation states, d-elements exhibit metallic properties; with an increase in the serial number in groups B, they decrease. In solutions, d-elements with the highest oxidation state exhibit acidic and oxidizing properties, and vice versa at lower oxidation states. Elements with an intermediate oxidation state exhibit amphoteric properties.
8. Covalent bond. Valence bond method
A chemical bond carried out by shared electron pairs arising in the shells of the bonded atoms having antiparallel spins is called atomic or covalent bond. The covalent bond is two-electron and two-center (holds nuclei). It is formed by atoms of one type - covalent non-polar– a new electron pair, which has arisen from two unpaired electrons, becomes common for two chlorine atoms; and atoms of different types, similar in chemical nature - covalent polar. Elements with greater electronegativity (Cl) will pull shared electrons away from elements with less electronegativity (H). Atoms with unpaired electrons that have parallel spins repel each other - no chemical bond occurs. The way a covalent bond is formed is called exchange mechanism.
Properties of a covalent bond. Link length - internuclear distance. The shorter this distance, the stronger the chemical bond. Bond energy - the amount of energy required to break the bond. The magnitude of the bond multiplicity is directly proportional to the bond energy and inversely proportional to the bond length. Direction of communication - a certain arrangement of electron clouds in a molecule. Saturability- the ability of an atom to form a certain number of covalent bonds. A chemical bond formed by the overlapping of electron clouds along an axis connecting the centers of atoms is called ?-connection. The bond formed by the overlapping of electron clouds perpendicular to the axis connecting the centers of atoms is called ?-bond. The spatial orientation of a covalent bond is characterized by the angles between the bonds. These angles are called valence angles. Hybridization - the process of rearrangement of electron clouds unequal in form and energy, leading to the formation of hybrid clouds identical in the same parameters. Valence is the number of chemical bonds (covalent ), through which an atom is connected to others. The electrons involved in the formation of chemical bonds are called valence. The number of bonds between atoms is equal to the number of its unpaired electrons involved in the formation of common electron pairs, so the valence does not take into account polarity and has no sign. In compounds in which there is no covalent bond, oxidation state - conditional charge of an atom, based on the assumption that it consists of positively or negatively charged ions. For most inorganic compounds, the concept of oxidation state is applicable.
Most compounds known on Earth are compounds of p-elements, five of them (C, N, P, O, S) are organogenic, that is, they are part of any cell. P-elements are in the main subgroups from III to VIII groups. Valence electrons are in the outer p-sublevel, the general electronic formula of the outer level corresponds to the composition ns 2 np a, where a \u003d 1 - 6. P-elements exhibit a positive oxidation state equal to the group number. In the nature of intermediate oxidation states, the “evenness rule” is manifested - the elements of odd groups show odd oxidation states, and the elements of even groups show even ones. Negative oxidation states appear in elements starting from the 4 A subgroup.
In periods from left to right, the atomic radii of the p-elements decrease, the ionization energy increases, which leads to an increase in the non-metallic and oxidizing properties of the p-elements. In subgroups in the direction from top to bottom, the metallic properties and stability of lower oxidation states increase.
Selenium, fluorine, bromine and iodine are trace elements and are found in the body in the form of ions with an oxidation state of 2 for selenium and -1 for halides. Ion Cl - is a macronutrient. P-elements in the lowest positive oxidation state exhibit a toxic effect, while in the highest they are trace elements.
The manual presents a brief description of the biological action of the most important P-elements.
Nitrogen - the main component of air: its volume fraction is 78.2%. The simplest nitrogen compounds are ammonia and ammonium salts, which are formed as a result of catabolism, as well as during the decomposition of plants and animal organisms. Ammonium ions cannot penetrate cell membranes, while ammonia molecules easily overcome membrane barriers and quickly affect the brain, which was previously used in medical practice for fainting. Ammonia is a toxic gas that, if inhaled, can attack the mucous membranes of the respiratory tract, cause shortness of breath and pneumonia.
Nitric oxide (II) NO can be formed in the atmosphere under the action of lightning discharges according to the equation:
N 2 + O 2 ¾® 2NO
In the late 1980s, it was found that NO is synthesized by endothelial cells using the enzyme NO synthase from the amino acid arginine. The lifetime of NO does not exceed a second, but the normal functioning of blood vessels without its participation is impossible. This compound provides relaxation of vascular smooth muscles, regulates the work of the heart, the immune system, is involved in the transmission of nerve impulses, and sexual arousal. NO is thought to play an important role in learning and memory. In 1988, the Nobel Prize was awarded for the discovery of the properties of NO (Furchgott, Ignarro, Murad).
Nitric oxide (IV) NO 2 is a strong oxidizing agent. It is formed from nitric oxide (ΙΙ) according to the equation 2NO + O 2 ¾® 2NO 2.
Nitric oxide NO 2 , which is released in large quantities during the combustion of fuel in power plants, can cause acid rain. Acid rain leads to a decrease in the pH of lakes and the death of fish, affects the structure of soils, which causes the death of crops and trees.
When nitrogen oxides are inhaled, nitric and nitrous acids are formed in the lungs, causing irritation, ulceration of the lungs, and with prolonged inhalation - tumors. The reactions of interaction of nitrogen oxides with water are given below
2NO 2 + H 2 O → HNO 3 + HNO 2
N 2 O 3 + H 2 O → 2HNO 2
N 2 O 4 + H 2 O → HNO 3 + HNO 2
N 2 O 5 + H 2 O → 2HNO 3
Nitrites (NO 2 -), used as preservatives for meat products, form nitrous acid HO-N=O, which nitrosates the amino groups of proteins with the formation of nitrosoamines according to the reaction:
R 2 N-H + HO-N=O ® R 2 N-N=O + H 2 O.
Nitrosamines give meat and sausage products a pink-red color. In high concentrations, nitrosamines exhibit a toxic effect and can cause bladder cancer. Nitrites can oxidize the Fe +2 cation (hemoglobin) to the Fe +3 cation (methemoglobin):
HbFe 2+ + NO 2 - ® HbFe +3 + NO
This is one of the reasons for the toxic effect of nitrites.
Nitrates (NO 3 -) present in food, entering the body, are easily reduced to toxic nitrites. A high content of nitrates in water can lead to stomach cancer (with low acidity), cause infant mortality. Nitrogen compounds are used in medicine as narcotic (nitrous oxide), diuretic (ammonium chloride), antiangial (nitroglycerin), antitumor (embichin), radioprotective (merkamin) means. Methylamine, dimethylamine, diethylamine and other aliphatic amines are used in the synthesis of drugs..
Phosphorus is an organogen, the total mass fraction of this macroelement in the human body is 0.95%. Phosphorus is found in bone tissue, kidneys, muscles, liver, blood, milk, hair, nails and teeth. Phosphates in living organisms serve as structural components of the skeleton. The rest of phosphoric acid is included in the structure of phospholipids of cell membranes, nucleic acids, complex carbohydrates. Polyphosphates (tri- and diphosphates) are involved in the accumulation of energy in the form of macroergic bonds (for example, ATP, creatine phosphate). About 30 g of ATP is present in the human body. The energy of ATP hydrolysis is the main energy currency that ensures the circulation of energy in the cell.
Phospholipids form the bilayer structure of biological membranes. Phosphorus in the form of phospholipids is mainly concentrated in the brain (12%), liver (5%), milk (2-3%) and blood serum (0.6%). However, the main amount of phosphorus - 600 g - is contained in inert tissue, which is 85% of the mass of all phosphorus in the human body. In the hard tissues of teeth, phosphorus is in the form of hydroxyl-, chlorine-, fluorapatites of the general formula Ca 5 (PO 4) 3 X, where X = OH, Cl, F, respectively. The main mineral component of bone tissue is calcium hydroxyphosphate Ca 5 (PO 4) 3 OH, called hydroxyapatite. The exchange of phosphorus in the body is closely related to the exchange of calcium, but this connection is antagonistic. With an increase in the calcium content in the blood, a decrease in the content of phosphates, primarily inorganic, is observed.
Phosphorus enters the body with food - milk, meat, fish, bread, vegetables, eggs, etc. The daily need for phosphorus is 0.8-1.2 g, excess phosphate contributes to the loss of manganese and calcium, which leads to osteoporosis.
In medicine, many phosphorus compounds are used in the form of drugs for the treatment of diseases of the heart, liver, and stomach. Zinc phosphates are used as filling materials in dentistry.
Oxygen refers to organogens. The body of an adult weighing 70 kg contains approximately 43 kg of oxygen. Together with hydrogen, oxygen forms a water molecule, the content of which in the body of an adult is on average about 55 - 65%.
Oxygen is a part of proteins, nucleic acids and other vital components of the body. Oxygen is essential for breathing. The exothermic oxidation reaction of biomolecules (fats, proteins, carbohydrates, amino acids) serves as a source of energy for the body. With the participation of oxygen (O 2), phagocytic (protective) functions of the body are carried out, as well as respiratory processes. The main amount of oxygen enters the body through the lungs, penetrates into the blood and, with the participation of hemoglobin, is delivered to all organs and tissues. Oxygen enters the body through the lungs, enters the bloodstream, binds to hemoglobin and forms an easily dissociating compound - oxyhemoglobin, and then from the blood enters all organs and tissues. Almost all oxygen is metabolized to carbon dioxide and water and excreted from the body through the lungs and kidneys.
Molecular oxygen (O 2) usually does not enter into direct non-enzymatic chemical reactions with organic compounds. The reaction involving O 2 in a living cell most often occurs in the active center of oxidase or oxygenase enzymes. In the course of these reactions, intermediate products of O 2 reduction are formed, which in the reaction center of enzymes undergo transformations to carbon dioxide and water. With the participation of a number of enzymes (xanthine oxidase), hemoglobin, intermediate products of oxygen reduction, the so-called reactive oxygen species (ROS), which are highly reactive, are generated in the body.
These include superoxide anion radicals (O 2), hydrogen peroxide (H 2 O 2), hydroxyl radicals (OH), as well as oxygen molecules in the singlet state (O 2 * ). (The ground state of oxygen molecules is triplet, which is characterized by the presence of two unpaired electrons with the same spin in different π * orbitals). The formation of ROS proceeds according to the scheme:
1. one-electron reduction of O 2 leads to the formation of a superoxide radical anion (O 2 ), which is the ancestor of other ROS:
O 2 + e → O 2
This reaction proceeds, in particular, during the oxidation of hemoglobin, while the electron released in the reaction is transferred to oxygen
Fe 2+ - e → Fe 3+
2. superoxide anion-radical, enters into a dismutation reaction regulated by the enzyme superoxide dismutase (SOD), resulting in the formation of hydrogen peroxide (H 2 O 2):
About 2 + O 2 + 2 H + → H 2 O 2 + O 2
3. The formation of a hydroxyl radical (OH) occurs when hydrogen peroxide interacts with a superoxide anion radical or metal ions of variable valence:
H 2 O 2 + O 2 → BUT + OH ─ + O 2
H 2 O 2 + Fe +2 → HO + OH ─ + Fe +3 (Fenton reaction)
The Fenton reaction reflects the toxic effect of hydrogen peroxide on hemoglobin, since the Fe +2 cation is oxidized to the Fe +3 cation, aggravated by the formation of a hydroxyl radical;
4. Singlent oxygen (O 2 *) is formed when an oxygen molecule in the triplet state is excited under the action of a light quantum (hυ). As a result, an electronic rearrangement of the molecule occurs, in which electrons with oppositely directed spins are located in one or different π * orbitals:
It is also possible to form O 2 * by the reaction between the superoxide anion radical and the hydroxyl radical:
O 2 + BUT → O 2 * + OH ─
ROS play an important role in the life of the body. For example, the radical anion superoxide is involved in the activation of phagocytic cells (neutrophils, macrophages, monocytes, eosinophils) necessary for the destruction of foreign microorganisms, tumor cells. ROS are involved in the processes of apoptosis (spontaneous death of cells, organs, or the organism as a whole).
The processes of ROS formation normally occur in the body and are regulated by antioxidant defense enzymes (superoxide dismutase, catalase, glutathione peroxidase, glutathione transferase).
catalase
2 H 2 O 2 H 2 O + O 2
superoxide dismutase
O 2 _ + O 2 _ + 2H + H 2 O 2 + O 2
glutathione peoxidase
R-SH + H 2 O 2 2 H 2 O + R-S-S-R
R-S-S-R + 2H + +2e 2 R-SH
An excessive amount of ROS leads to the development of a number of pathological conditions, which are based on oxidative transformations of lipids in biological membranes, damage to the structure of nucleic acids, proteins and their supramolecular complexes. These transformations, conditionally in a general form, are represented by the scheme:
RH + OH ∙ → R ∙ + H 2 O
R ∙ + O 2 → RO 2 ∙
RH + RO 2 ∙ → ROOH + R ∙
Strengthening free radical oxidation processes leads to disruption of the integrity of biological membranes and cell death, causes changes in the structure of proteins, a decrease in enzyme activity, and is the cause of mutations.
In medicine, molecular oxygen is used to treat hypoxic conditions, cardiovascular diseases, cyanide and carbon monoxide poisoning. Dosed exposure to oxygen is carried out at elevated pressure (hyperbaric oxygenation), resulting in improved hemodynamics and oxygen supply to tissues. In cardiovascular diseases, oxygen foam (oxygen cocktail) is used to improve metabolic processes. Subcutaneous administration of oxygen (ozone) is indicated for trophic ulcers, gangrene. Ozonation of drinking water is used for its purification and disinfection.
Carbon is the most important organogen. The total carbon content is about 21% (15 kg per 70 kg of total body weight). Carbon makes up 2/3 of muscle mass and 1/3 of bone mass. The physiological role of carbon is determined by the fact that this element is part of all organic compounds and takes part in all biochemical processes in the body. Oxidation of biomolecules under the action of oxygen leads to the formation of water and carbon dioxide (CO 2), which is a stimulator of the respiratory center, plays an important role in the regulation of respiration and blood circulation.
In free form, carbon is not toxic, but many of its compounds have significant toxicity: CO (carbon monoxide), carbon tetrachloride CCI 4, carbon disulfide CS 2, cyanide salts HCN, benzene C 6 H 6, phosgene COCI 2, and a number of others. Carbon dioxide in concentrations above 10% causes acidosis (decrease in blood pH), shortness of breath and paralysis of the respiratory center. In pharmacy and medicine, various carbon compounds are widely used - derivatives of carbonic acid and carboxylic acids, polymers, etc. Carbolene (activated carbon) is used to adsorb gases and remove various toxins from the body, graphite in the form of ointments is used to treat skin diseases. In biomedical research, products labeled with 14 C are used.
Sulfur refers to macroelements, organogens. Sulfur is involved in the formation of compounds in the -2 oxidation state. In the form of sulhydryl - SH - groups or disulfide bonds - S - S - sulfur is part of proteins, amino acids (cysteine, cystine, methionine), hormones (insulin), enzymes (coenzyme A), vitamins (B1), keratin (hair , bones, nervous tissue). The tertiary structure of the protein contains disulfide bridges between cystine amino acid residues. The reversible transition of thiol groups into disulfide bonds protects the body from radiation damage and the action of strong oxidizing agents:
R 1 - S- S-R 2 R 1 - SH + R 2 - SH
R-S – H R-S – H S-R 1
Pb2+ → Pb2+
R 1 -S - H R 1 -S H-S-R
As a result, the enzyme loses its activity and the course of biochemical reactions is disturbed. In the process of metabolism of sulfur compounds, endogenous sulfuric acid is formed, which is involved in the neutralization of toxic compounds (phenol, indole) produced in the intestine by microorganisms. Sulfuric acid binds many xenobiotics into relatively harmless substances (conjugates) that are excreted in the urine.
Toxic sulfur compounds are H 2 S hydrogen sulfide, SO 2 sulfur dioxide. H 2 S hydrogen sulfide is contained in sulfurous mineral waters, which are used in the form of baths to treat a number of diseases. It is a colorless gas with an unpleasant odor. It is formed during the decay of plant and animal residues under the action of microorganisms.
SO 2 - sulfur dioxide, has a suffocating smell. Poisonous. Acts as an irritant to the mucous membrane of the respiratory tract. Approximately one third of sulfur oxide (IV) enters the atmosphere due to the microbiological oxidation of organic substances, its source is active volcanoes. About 70% of SO 2 is formed as a result of combustion of oil products and sulfurous ores. Under the action of sunlight and catalysts (V 2 O 5), sulfur oxide SO 2 turns into SO 3:
2SO 2 + O 2 → 2SO 3
Being dissolved in atmospheric moisture, SO 3 forms sulfuric acid, which forms acid rain, leading to the death of forests, acidification of the soil.
SO 3 (g) + H 2 O (l) → H 2 SO 4 (aq)
Sodium thiosulfate Na 2 S 2 O 3 is used in medical practice as an anti-toxic, anti-inflammatory agent for poisoning with mercury compounds, lead, hydrocyanic acid salts. Sodium thiosulfate and precipitated sulfur are used in the treatment of scabies.
Sulphates of many metals are used as medicines: Na 2 SO 4 ´10H 2 O - as a laxative, MgSO 4 ´7H 2 O - as a laxative and choleretic agent, CuSO 4 ´5H 2 O and ZnSO 4 ´7H 2 O as antiseptic, astringents, emetics. BaSO 4 is used as a contrast agent in x-ray examination of the esophagus and stomach. Sulfur precipitated is used in the treatment of scabies.
Sulfur enters the body with food. The richest in sulfur compounds are eggs, meat, cottage cheese, buckwheat, bran, wholemeal bread.
Chlorine in the human body is contained in the amount of 100 g (0.15%) mainly in the form of chloride ion. The chloride ion has an optimal radius for penetration through the cell membrane. This explains its joint participation with sodium and potassium ions in the creation of a certain osmotic pressure and the regulation of water-salt metabolism. The daily requirement for sodium chloride is 1 g of NaCl, which is necessary for the production of hydrochloric acid (hydrochloric acid) in the stomach, which plays an important role in the digestion process and destroys various pathogenic bacteria (cholera, typhoid).
Vital chloride - ions do not have a toxic effect, while elemental chlorine is a highly toxic gas.
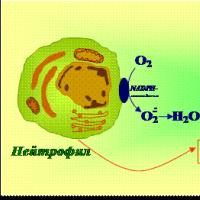
In recent years, the formation in the body of a number of active forms of halogens (AHS) has been established - halogen-containing compounds with increased reactivity and formed in a living organism or entering it as a result of human contact with the environment.
Reactive forms of halogens (AFHs) are halogen-containing compounds that have an increased reactivity and are formed in a living organism or enter it as a result of human contact with the environment. Distinguish between exogenous (introduced) and endogenous (formed in the body) AFG. Exogenous sources of active forms of halogens include pesticides, pharmaceuticals, anesthetics, wastewater, car and aircraft exhaust gases, and industrial poisons. Active forms of halogens are formed in the body with the participation of peroxidase enzymes, in particular myeloperoxidase, as well as H 2 O 2 reductase, mainly in neutrophils. Hypochlorous HOCl and hypobromous HOCl acids (primary APBs) are formed from chloride and bromide ions, which can become sources for the formation of active chlorine and bromine, as well as halogenation products of the most important biomolecules: amino acids, lipids, nucleic acids, cholesterol (secondary APBs) (see Fig. scheme).
Cl 2 + H 2 O → H + + Сlˉ + HOCl

Active forms of halogens in small quantities are necessary for the destruction and neutralization of microorganisms; if they are in excess, they can serve as sources of active free radicals that have a damaging effect on body structures.
In cases where the appearance or formation of AFG exceeds the body's ability to remove or neutralize these compounds, numerous pathologies can develop, including atherosclerosis, heart attacks, strokes, vasculitis, Alzheimer's disease, respiratory, renal dysfunction, rheumatoid arthritis, sepsis, etc.
Selenium is a trace element, mainly concentrated in the liver and kidneys. The concentration of selenium in the blood is 0.001 - 0.004 mmol / l.
In living organisms, the connection of selenium with sulfur is undeniable. At high doses, selenium primarily accumulates in nails and hair, which are based on sulfur-containing amino acids. Obviously, selenium, as an analogue of sulfur, replaces it in various compounds:
R- S- S- R ¾® R- Se-Se- R
It has been established that the lack of selenium leads to a decrease in the concentration of the enzyme glutathione peroxidase, which, in turn, leads to the oxidation of lipids and sulfur-containing amino acids.
Studies conducted in recent years have shown that selenium in combination with any acid is part of the active centers of several enzymes: formate dehydrogenase, glutathione reductase and glutathione peroxidase, glutathione transferase. In particular, the active center of glutathione peroxidase contains a residue of the unusual amino acid selenocysteine: HOOC-CH(NH 2)-CH 2 -Se-H. This enzyme, together with the protein glutathione, protects cells from the damaging effects of organic peroxides ROOH and hydrogen peroxide H 2 O 2 . It is possible that the hydrogen selenide group - SeH of selenocysteine has some advantages over the hydrogen sulfide group -SH in the mechanism of action of this and other selenium containing enzymes.
The ability of selenium to protect the body from poisoning with salts of mercury Hg and cadmium Cd is well known. It turned out that selenium promotes the binding of salts of these toxic metals with the active centers of other enzymes, which are not affected by their toxic effect.
It is shown that selenium stimulates the formation of antibodies and thereby increases the body's defense against infectious and colds. Participates in the production of red blood cells, helps maintain sexual activity. In the male body, almost 50% of selenium is concentrated in the seminiferous tubules of the testicles, selenium is lost with the ejaculate. Therefore, the requirement of men for selenium is higher than for women. The activity of selenium is increased in the presence of vitamin E. The fact of the relationship between a high content of selenium in the diet and low mortality from cancer has been established.
Selenium is toxic in high doses. The breakdown of selenium compounds in animals leads to the release of highly toxic dimethyl selenium CH 3 -Se-CH 3 , which has a garlic odor. The mechanism of this reaction has been established. When selenous acid H 2 SeO 3 reacts with glutathione, compounds containing the -S-Se-S- group are formed
H 2 SeO 3 + 4GSH ¾® GSSeSG + GSSG + 3H 2 O
reduced oxidized
glutathione glutathione
Under the action of enzymes, compounds containing a group
S-Se-S- are reduced to hydrogen selenide H 2 Se, which is methylated to form toxic dimethyl selenium.
The diet of most industrialized countries is deficient in trace elements. The need for an adult is 150-200 mcg / day. Contained in meat, liver, kidneys, marine fish, yeast, bread, Jerusalem artichoke. However, additional sources of selenium are often required, which are vitamin-mineral complexes and other biologically active food supplements.
Selenium compounds (sodium selenite, selenium-methionine, selenium-cysteine, etc.) are widely used in medicine for the treatment and prevention of many diseases, since selenium is a natural antioxidant. In dermatology and cosmetology, selenium-containing shampoos, creams, soaps and gels are used. The isotope 15 Se in the composition of sodium selenate and selenite is used in medical research.
Iodine is one of the essential micronutrients. The human body contains about 25 mg (4 10 -5%) of iodine, most of it is in the thyroid gland in the structure of hormones (triiodothyronine, thyroxine). In the form of iodide ion I - is about 1% of the iodine present in the body.
The main source of iodine for the human body is seafood, as well as iodophors and iodized salt used in the food industry. The amount of iodine in fruits and vegetables depends on the composition of the soil, as well as on the type of food processing. The thyroid gland is able to concentrate iodine, the content of the element in it is 25 higher than in blood plasma. The thyroid gland secretes the hormones thyroxine and triiodothyronine. There is evidence that iodine affects the synthesis of certain proteins, fats, hormones.
An underactive thyroid gland (hypothyroidism) may be associated with a decrease in its ability to accumulate iodide ions, as well as a lack of iodine in the diet (endemic goiter). With endemic goiter, iodine preparations are prescribed: potassium iodide KI or sodium iodide NaI in doses corresponding to the daily human need for iodine (0.00l g of potassium iodide). In areas where iodine deficiency is noted, NaI or KI (I.0 - 2.5) g / kg of salt is added to table salt to prevent endemic goiter).
With increased activity of the thyroid gland (hyperthyroidism), due to excessive synthesis of thyroid hormones, an increase in the rate of metabolic processes is observed.
NaI and KI are used in inflammatory diseases of the respiratory tract. Iodine preparations are used externally as antiseptics (for example, iodoform), as irritants and distractions in inflammatory diseases of the skin and mucous membranes. Preparations containing iodine include: 5% alcohol solution of iodine, anti-asthma mixture, potassium and sodium iodides, calcium odin, antistrumine and iodactiv tablets.
Fluorine is a micronutrient. Fluorine compounds are concentrated in bone tissue, nails, teeth. The composition of the teeth includes about 0.01% fluorine, and most of it falls on the enamel, which is associated with the presence of sparingly soluble fluorapatite Ca 3 (PO 4) 3 F in it. A lack of fluorine in the body leads to tooth decay. The mineral basis of dental tissues - dentin is made up of hydroxyapatite Ca 5 (PO 4) 3 (OH), chlorapatite Ca 5 (PO 4) 3 C1 and fluorapatite Ca 5 (PO 4) 3 F. Fluoride ion easily replaces the hydroxide ion in hydroxyapatite, forming protective enamel layer of harder fluorapatite:
Ca 10 (PO 4) 6 (OH) 2 + F ‾ ¾® Ca 10 (P0 4) F 2 + 2 OH ‾
In addition, fluoride ions contribute to the precipitation of calcium phosphate, accelerating the process of remineralization (crystal formation):
1O Ca 2+ + 6PO 4 ‾3 + 2F ‾ ¾® 3Ca 3 (PO 4) 2 + CaF 2
Dental caries is a process of dissolution of the hydroxyapatite component of enamel under the action of acids produced by bacteria:
Ca 5 (PO 4) 3 OH + 7H + ¾® 5Ca 2+ + 3H 2 PO 4 - + H 2 O
There are suggestions that with a slight damage to the enamel, the introduction of sodium fluoride promotes the formation of fluorapatite, which facilitates the remineralization of the damage that has begun. Fluoridation of water with sodium fluoride (up to the content of fluoride ions of 1 mg/l) leads to a significant reduction in the incidence of dental caries in the population.
Sodium fluoride is used in medical practice as a topical external agent. The use of NaF is based on the formation of fluorapatite:
NaF + Ca 10 (PO 4) 6 (OH) 2 ¾® NaOH + Ca 10 (PO 4) 6 F 2
tooth tissue paste
At the same time, alkalization of the oral cavity environment and neutralization of acids produced by bacteria occur simultaneously.
Fluorine compounds enter the body with food and water. A lot of fluoride in rice, beef, eggs, milk, onions, spinach, apples.
Not only a lack, but also an excess of fluorine is harmful. When the fluorine content in drinking water is above the maximum allowable rate (1.2 mg/l), tooth enamel becomes brittle, easily destroyed, and other symptoms of chronic fluorine poisoning appear - increased bone fragility, bone deformities, and general exhaustion of the body. The disease that occurs in this case is called fluorosis (fluorosis).
Bromine - trace element. The mass of bromine in the human body is about 7 mg (~10 -5%). The biological role of bromine compounds is not well understood. It is localized in the endocrine glands, primarily in the pituitary gland, kidneys, thyroid gland, interstitial fluid. The increased content of bromide anions contributes to the excretion of chloride anions by the kidneys. There is evidence that bromine compounds inhibit the function of the thyroid gland and increase the activity of the adrenal cortex. The most sensitive to the introduction of bromide ions into the body is the central nervous system. Bromides accumulate in various parts of the brain, enhancing inhibitory processes in the neurons of the cortex, so bromine preparations (potassium, sodium, bromocamphor bromides) are used as sedatives in case of increased excitability, help to restore the disturbed balance between the processes of excitation and inhibition
In terms of ionic radius, electronegativity and other physicochemical characteristics, bromine occupies an intermediate position between chlorine and iodine. Therefore, bromide ions can replace C1 - and I - ions in the body. An example of such mutual substitution is the replacement of iodine with bromine when there is an excess of bromine in the body in thyroid hormones, which leads to hyperthyroidism.
Due to different individual sensitivities, the dosage of bromine preparations varies from 0.05 to 2.0 g. Bromine enters the body with cereals, nuts and fish.
Bor . It has long been known that the trace element boron is necessary for higher plants, however, data on its biological role have appeared relatively recently - since 1985. It has been established that boron is involved in carbon-phosphate metabolism, interacts with a number of biologically active compounds (carbohydrates, enzymes, vitamins, hormones) . It has been established that boron is a partner of silicon, calcium, manganese, magnesium involved in the processes of calcification, bone formation and prevention of osteoporosis. In the mechanism of its influence on calcium metabolism in postmenopausal women, an important role is played by an increase in the level of active estrogens. Boron is involved in the activation of both estrogen and vitamin D. Under the influence of boron, calcium excretion in the urine decreases and the level of 17-β-estradiol increases. Boron preparations prevent the loss of calcium in the urine, which is important for osteoporosis and fractures. Boron, together with zinc, is involved in the mobilization of fatty acids from fat cells. Boron preparations relieve joint pain and improve well-being. The most effective and safe are organic derivatives of the trace element, for example, boron glycerinate. Inorganic derivatives - boric acid and borax can have a toxic effect. Borax - hydrated sodium tetraborate Na 2 B 4 O 7 10H 2 O is widely used as an antiseptic. The pharmacological action of the drug is due to the hydrolysis of salt with the release of boric acid:
Na 2 B 4 O 7 + 7H 2 O ¾® 4H 3 BO 3 + 2NaOH
The resulting alkali and acid cause coagulation (denaturation) of microbial cell proteins.
In dental prosthetics, boric acid H 3 BO 3 is used as a mold filler when casting dentures. The composition of dental pastes used as an adhesive layer for dentures includes sodium metaborate NaB0 2 mixed with aluminum hydroxide A1 (OH) 3.
The daily requirement for boron is approximately 2-7 mg. Boron sources are fruits, vegetables, nuts, wines.
The use of food products with a high content of boron disrupts the metabolism of carbohydrates and proteins in the body, which leads to the occurrence of endemic intestinal diseases - enteritis.
Aluminum is an immunotoxic trace element. The human body contains 10-5% of aluminum and daily comes from 5 to 50 mg. The source of aluminum intake is food and drinking water. With age, the content of this element in the lungs and brain increases. Aluminum is involved in the formation of phosphate and protein complexes, the processes of regeneration of bone, connective and epithelial tissue, has an inhibitory or activating effect (depending on concentration) on digestive enzymes, and affects the function of the parathyroid glands.
In medicine, the adsorbing, enveloping, antacid, protective and analgesic properties of preparations containing aluminum are used. Aluminum silicate (white clay, kaolin) and burnt alum KAI(SO 4) 3 7H 2 O are used externally in the form of powders, ointments and pastes in the treatment of skin diseases. AI(OH) 3 is used as an antacid for gastric and duodenal ulcers, gastritis and poisoning. AI (OH) 3 together with MgO is part of the drug "Almagel" used as an enveloping and antacid agent for stomach diseases. Aluminum phosphate has an antiulcer, adsorbing effect, reduces the acidity of gastric juice.
Arsenic - immunotoxic microelement, contained in the human body in an amount (10 -6%). Arsenic accumulates in the bones and hair and is not completely removed from them for several years. This feature is used in forensic examination to clarify the question of whether there has been poisoning with arsenic compounds.
Arsenic compounds enter the human body with drinking and mineral water, grape wines and juices, seafood, medicines, pesticides and herbicides. Arsenic can enter the body in increased amounts with atmospheric air, tk. its concentration in the air increases when coal is burned in boiler houses and thermal power plants, near copper smelters. In drinking water in some regions of the world (India, Bangladesh, Taiwan, Mexico), the content of arsenic is increased (1 mg / l), which is the cause of massive chronic arsenic poisoning and causes the so-called "black foot" disease. Arsenic (V) compounds and especially arsenic (III) compounds are very toxic. The mechanism of toxic action is explained by the ability of arsenic to block sulfhydryl SH - groups of enzymes, proteins, amino acids (cysteine, glutathione, lipoic acid).
In addition, arsenic can replace iodine, selenium and phosphorus, disrupting the biochemical processes of metabolism in the body, As is an antimetabolite of these elements. The lethal dose for humans is approximately 0.1-0.3 g of arsenic.