efficiency of heat engines. Heat engine efficiency - formula
Modern realities involve the widespread operation of heat engines. Numerous attempts to replace them with electric motors have so far failed. The problems associated with the accumulation of electricity in autonomous systems are solved with great difficulty.
Still relevant are the problems of technology for the manufacture of electric power accumulators, taking into account their long-term use. The speed characteristics of electric vehicles are far from those of cars on internal combustion engines.
The first steps towards the creation of hybrid engines can significantly reduce harmful emissions in megacities, solving environmental problems.
A bit of history
The possibility of converting steam energy into motion energy was known in antiquity. 130 BC: Philosopher Heron of Alexandria presented to the audience a steam toy - aeolipil. A sphere filled with steam began to rotate under the action of jets emanating from it. This prototype of modern steam turbines did not find application in those days.
For many years and centuries, the development of the philosopher was considered only a fun toy. In 1629, the Italian D. Branchi created an active turbine. Steam set in motion a disk equipped with blades.
From that moment began the rapid development of steam engines.
heat engine
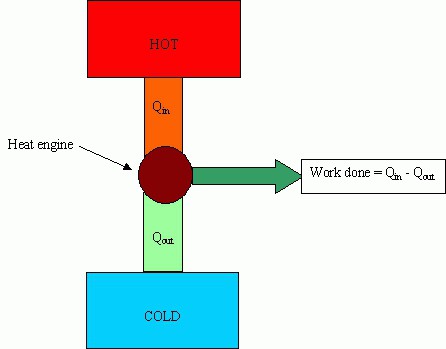
The conversion of fuel into energy for the movement of parts of machines and mechanisms is used in heat engines.
The main parts of machines: a heater (a system for obtaining energy from outside), a working fluid (performs a useful action), a refrigerator.
The heater is designed to ensure that the working fluid has accumulated a sufficient supply of internal energy to perform useful work. The refrigerator removes excess energy.
The main characteristic of efficiency is called the efficiency of heat engines. This value shows what part of the energy spent on heating is spent on doing useful work. The higher the efficiency, the more profitable the operation of the machine, but this value cannot exceed 100%.
Efficiency calculation
Let the heater acquire from outside the energy equal to Q 1 . The working fluid did work A, while the energy given to the refrigerator was Q 2 .
Based on the definition, we calculate the efficiency:
η= A / Q 1 . We take into account that A \u003d Q 1 - Q 2.
From here, the efficiency of the heat engine, the formula of which has the form η = (Q 1 - Q 2) / Q 1 = 1 - Q 2 / Q 1, allows us to draw the following conclusions:
- Efficiency cannot exceed 1 (or 100%);
- to maximize this value, either an increase in the energy received from the heater or a decrease in the energy given to the refrigerator is necessary;
- an increase in the energy of the heater is achieved by changing the quality of the fuel;
- reducing the energy given to the refrigerator, make it possible to achieve the design features of the engines.

Ideal heat engine
Is it possible to create such an engine, the efficiency of which would be maximum (ideally, equal to 100%)? The French theoretical physicist and talented engineer Sadi Carnot tried to find the answer to this question. In 1824, his theoretical calculations about the processes occurring in gases were made public.
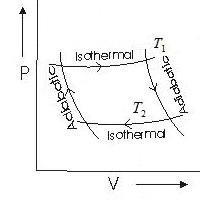
The main idea behind an ideal machine is to carry out reversible processes with an ideal gas. We start with the expansion of the gas isothermally at a temperature T 1 . The amount of heat required for this is Q 1. After the gas expands without heat exchange. Having reached the temperature T 2, the gas is compressed isothermally, transferring the energy Q 2 to the refrigerator. The return of the gas to its original state is adiabatic.
The efficiency of an ideal Carnot heat engine, when accurately calculated, is equal to the ratio of the temperature difference between the heating and cooling devices to the temperature that the heater has. It looks like this: η=(T 1 - T 2)/ T 1.
The possible efficiency of a heat engine, the formula of which is: η= 1 - T 2 / T 1 , depends only on the temperature of the heater and cooler and cannot be more than 100%.
Moreover, this ratio allows us to prove that the efficiency of heat engines can be equal to unity only when the refrigerator reaches temperatures. As you know, this value is unattainable.
Carnot's theoretical calculations make it possible to determine the maximum efficiency of a heat engine of any design.
The theorem proved by Carnot is as follows. An arbitrary heat engine under no circumstances is capable of having a coefficient of efficiency greater than the similar value of the efficiency of an ideal heat engine.
Example of problem solving
Example 1 What is the efficiency of an ideal heat engine if the heater temperature is 800°C and the refrigerator temperature is 500°C lower?
T 1 \u003d 800 o C \u003d 1073 K, ∆T \u003d 500 o C \u003d 500 K, η -?
By definition: η=(T 1 - T 2)/ T 1.
We are not given the temperature of the refrigerator, but ∆T = (T 1 - T 2), from here:
η \u003d ∆T / T 1 \u003d 500 K / 1073 K \u003d 0.46.
Answer: efficiency = 46%.
Example 2 Determine the efficiency of an ideal heat engine if 650 J of useful work is performed due to the acquired one kilojoule of heater energy. What is the temperature of the heat engine heater if the coolant temperature is 400 K?
Q 1 \u003d 1 kJ \u003d 1000 J, A \u003d 650 J, T 2 \u003d 400 K, η -?, T 1 \u003d?
In this problem, we are talking about a thermal installation, the efficiency of which can be calculated by the formula:
To determine the temperature of the heater, we use the formula for the efficiency of an ideal heat engine:
η \u003d (T 1 - T 2) / T 1 \u003d 1 - T 2 / T 1.
After performing mathematical transformations, we get:
T 1 \u003d T 2 / (1- η).
T 1 \u003d T 2 / (1- A / Q 1).
Let's calculate:
η= 650 J / 1000 J = 0.65.
T 1 \u003d 400 K / (1- 650 J / 1000 J) \u003d 1142.8 K.
Answer: η \u003d 65%, T 1 \u003d 1142.8 K.
Real conditions
The ideal heat engine is designed with ideal processes in mind. Work is done only in isothermal processes, its value is defined as the area bounded by the Carnot cycle graph.
In fact, it is impossible to create conditions for the process of changing the state of a gas without accompanying changes in temperature. There are no materials that would exclude heat exchange with surrounding objects. The adiabatic process is no longer possible. In the case of heat transfer, the temperature of the gas must necessarily change.
The efficiency of heat engines created in real conditions differ significantly from the efficiency of ideal engines. Note that the processes in real engines are so fast that the variation in the internal thermal energy of the working substance in the process of changing its volume cannot be compensated by the inflow of heat from the heater and return to the cooler.
Other heat engines
Real engines operate on different cycles:
- Otto cycle: the process at a constant volume changes adiabatically, creating a closed cycle;
- Diesel cycle: isobar, adiabat, isochor, adiabat;
- the process occurring at constant pressure is replaced by an adiabatic one, closing the cycle.
It is not possible to create equilibrium processes in real engines (to bring them closer to ideal ones) under the conditions of modern technology. The efficiency of thermal engines is much lower, even taking into account the same temperature regimes as in an ideal thermal installation.
But you should not reduce the role of the efficiency calculation formula, since it is it that becomes the starting point in the process of working to increase the efficiency of real engines.
Ways to change efficiency
When comparing ideal and real heat engines, it is worth noting that the temperature of the refrigerator of the latter cannot be any. Usually the atmosphere is considered to be a refrigerator. The temperature of the atmosphere can be taken only in approximate calculations. Experience shows that the temperature of the coolant is equal to the temperature of the exhaust gases in the engines, as is the case in internal combustion engines (abbreviated internal combustion engines).
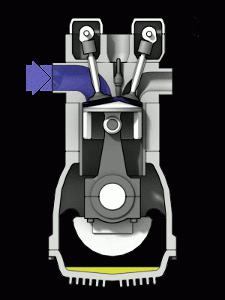
ICE is the most common heat engine in our world. The efficiency of a heat engine in this case depends on the temperature created by the burning fuel. An essential difference between an internal combustion engine and steam engines is the merging of the functions of the heater and the working fluid of the device in the air-fuel mixture. Burning, the mixture creates pressure on the moving parts of the engine.
An increase in the temperature of the working gases is achieved by significantly changing the properties of the fuel. Unfortunately, it is not possible to do this indefinitely. Any material from which the combustion chamber of an engine is made has its own melting point. The heat resistance of such materials is the main characteristic of the engine, as well as the ability to significantly affect the efficiency.
Motor efficiency values
If we consider the temperature of the working steam at the inlet of which is 800 K, and the exhaust gas is 300 K, then the efficiency of this machine is 62%. In reality, this value does not exceed 40%. Such a decrease occurs due to heat losses during heating of the turbine casing.
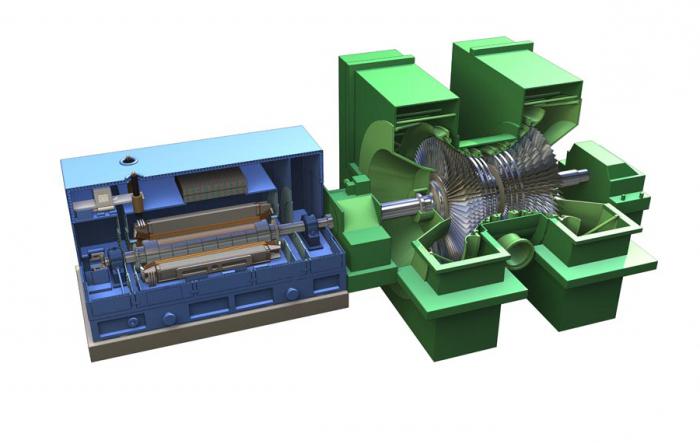
The highest value of internal combustion does not exceed 44%. Increasing this value is a matter of the near future. Changing the properties of materials, fuels is a problem that the best minds of mankind are working on.