What is the amount of a substance and how is it determined
Let's talk about what such a quantity of substance as this term is used in the subjects of the natural science cycle. Since serious attention is paid to quantitative relations in chemistry and physics, it is important to know the physical meaning of all quantities, their units of measurement, and areas of application.
Designation, definition, units of measurement
In chemistry, quantitative relationships are of particular importance. Special quantities are used to perform calculations according to equations. In order to understand what the amount of a substance in chemistry is, let's define the term. which characterizes the number of similar structural units (atoms, ions, molecules, electrons) present in the substance. To understand what the amount of a substance is, we note that this quantity has its own designation. When carrying out calculations involving the use of this value, use the letter n. Units of measurement - mol, kmol, mmol.
The value of the quantity
Eighth graders who do not yet know how to write chemical equations do not know what the amount of a substance is, how to use this quantity in calculations. After getting acquainted with the law of constancy of the mass of substances, the meaning of this quantity becomes clear. For example, in the combustion reaction of hydrogen in oxygen, the ratio of reactants is two to one. If the mass of hydrogen that entered the process is known, it is possible to determine the amount of oxygen that took part in the chemical reaction.
The use of formulas for the amount of a substance makes it possible to reduce the ratio between the initial reagents and simplify calculations. What is the amount of a substance in chemistry? From the point of view of mathematical calculations, these are the stereochemical coefficients put in the equation. They are used to carry out certain calculations. Since it is inconvenient to count the number of molecules, it is Mole that is used. Using it, one can calculate that 1 mol of any reagent includes 6 1023 mol −1 .

Computing
Do you want to understand what the amount of a substance is? In physics, this quantity is also used. It is needed where pressure and volume of gaseous substances are calculated according to the Mendeleev-Clapeyron equation. To perform any quantitative calculations, the concept is applied
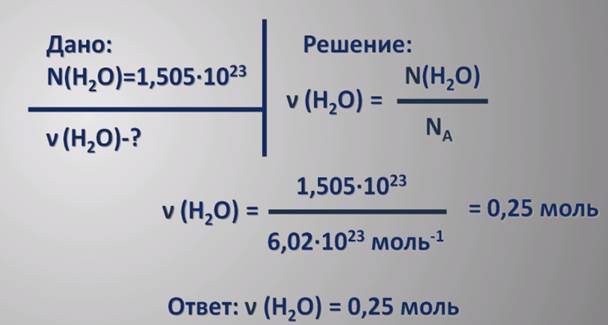
By it is meant the mass that corresponds to one mole of a particular chemical substance. You can determine the molar mass through (their sum, taking into account the number of atoms in the molecule) or determine through the known mass of the substance, its amount (mol).
Not a single task of a school chemistry course related to calculations according to an equation is complete without the use of such a term as “amount of substance”. Knowing the algorithm, you can cope not only with ordinary software calculations, but also with complex Olympiad tasks. In addition to calculations through the mass of a substance, it is also possible, using this concept, to carry out calculations through the molar volume. This is relevant in cases where gaseous substances are involved in the interaction.