Substitution reaction of benzene with nitric acid. Chemical properties of benzene and its homologues. Obtaining and using benzene
Among the various reactions that aromatic compounds enter into with the participation of the benzene ring, the substitution reactions discussed above attract attention first of all. This happens because they proceed contrary to expectations. With the degree of unsaturation that is inherent, for example, in benzene, addition reactions should have been more characteristic of this hydrocarbon. Under certain conditions, this happens, benzene and other arenes add hydrogen atoms, halogens, ozone and other reagents that can add.
11.5.5. Hydrogenation. In the presence of hydrogenation catalysts (platinum, palladium, nickel), benzene and its homologues add hydrogen and turn into the corresponding cyclohexanes. So, benzene is hydrogenated over a nickel catalyst at 100-200 0 C and 105 atm .:
Hydrogenation of arenes has two peculiarities compared to alkenes. Firstly, arenes are significantly inferior to alkenes in reactivity. For comparison with the conditions for the hydrogenation of benzene, we point out that cyclohexene is hydrogenated into cyclohexane already at 25 0 C and a pressure of 1.4 atm. Secondly, benzene either does not add, or attaches three hydrogen molecules at once. It is not possible to obtain partial hydrogenation products, such as cyclohexene or cyclohexadiene, by hydrogenation of benzene.
These features during hydrogenation, a special case of addition reactions to the benzene ring, are due to the structure of benzene. Upon conversion to cyclohexane, benzene ceases to be an aromatic system. Cyclohexane contains 150.73 kJ more energy (resonance energy) and is less stable than benzene. It is clear that benzene is not inclined to pass into this thermodynamically less stable state. This explains the lower reactivity of benzene with respect to hydrogen compared to alkenes. Accession to the aromatic system is possible only with the participation R-electrons of a single electron cloud of the benzene ring. When the addition process begins, the system ceases to be aromatic and a particle rich in energy and highly reactive is obtained, which is much more likely to enter into the addition reaction than the original arene.
11.5.6. Halogenation. The result of the interaction of halogen with benzene depends on the experimental conditions. Catalytic halogenation leads to the formation of substitution products. It turned out that ultraviolet light initiates the addition of halogen atoms to the benzene nucleus of arenes. Benzene itself in the light attaches 6 chlorine atoms and turns into hesachlorocyclohexane, which is a mixture of 9 spatial isomers
One of these isomers, in which 3 chlorine is occupied by axial bonds, and another 3 - by equatorial bonds (γ-isomer, hexachlorane), turned out to be an effective insecticide, a means of controlling harmful insects. Hexachlorane proved to be too stable in the biosphere and capable of accumulating in the adipose tissue of warm-blooded animals, and therefore is not currently used.
In terms of its reactivity with respect to halogens in addition reactions, benzene is significantly inferior to alkenes. For example, chlorine and bromine in carbon tetrachloride, even in the dark at room temperature, add to cyclohexene. Under these conditions, benzene does not react. This happens only under ultraviolet light.
11.5.7. Ozonation. Ozonation is another example showing that benzene, as an unsaturated compound, can enter into an addition reaction. The ozonation of benzene and the study of triozonide hydrolysis products were carried out as early as 1904 ( Harries)
Interesting results were obtained with ozonation about-xylene (1941, Vibo). The fact is that the composition of ozonation products depends on the position of double bonds in the benzene ring. Structure 1 with double bonds between the carbons of the benzene ring bearing methyl substituents, upon ozonation and hydrolysis of the ozonide, will give 2 molecules of methylglyoxal and a molecule of glyoxal
Alternative structure II for about-xylene would have to form 2 glyoxal molecules and a diacetyl molecule
The first group of reactions is substitution reactions. We said that arenes do not have multiple bonds in the molecular structure, but contain a conjugated system of six electrons, which is very stable and gives additional strength to the benzene ring. Therefore, in chemical reactions the replacement of hydrogen atoms occurs first, and not the destruction of the benzene ring.
We have already encountered substitution reactions when talking about alkanes, but for them these reactions proceeded according to a radical mechanism, while arenes are characterized by an ionic mechanism of substitution reactions.
First chemical property - halogenation. Substitution of a hydrogen atom for a halogen atom - chlorine or bromine.
The reaction proceeds when heated and always with the participation of a catalyst. In the case of chlorine, it can be aluminum chloride or iron chloride three. The catalyst polarizes the halogen molecule, resulting in heterolytic bond breaking and ions are obtained.
The positively charged chloride ion reacts with benzene.
If the reaction occurs with bromine, then iron tribromide or aluminum bromide acts as a catalyst.

It is important to note that the reaction occurs with molecular bromine and not with bromine water. Benzene does not react with bromine water.
The halogenation of benzene homologues has its own characteristics. In the toluene molecule, the methyl group facilitates substitution in the ring, the reactivity increases, and the reaction proceeds under milder conditions, that is, already at room temperature.

It is important to note that the substitution always occurs in the ortho and para positions, so a mixture of isomers is obtained.
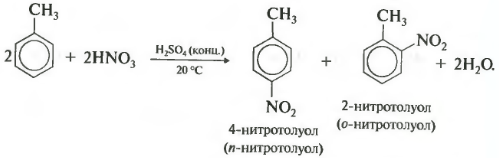
Second property - nitration of benzene, the introduction of a nitro group into the benzene ring.

A heavy yellowish liquid with the smell of bitter almonds is formed - nitrobenzene, so the reaction can be qualitative for benzene. For nitration, a nitrating mixture of concentrated nitric and sulfuric acids is used. The reaction is carried out by heating.
Let me remind you that for the nitration of alkanes in the Konovalov reaction, dilute nitric acid was used without the addition of sulfuric acid.
In the nitration of toluene, as well as in the halogenation, a mixture of ortho- and para-isomers is formed.
Third property - alkylation of benzene with haloalkanes.
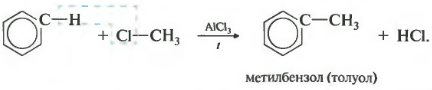
This reaction allows the introduction of a hydrocarbon radical into the benzene ring and can be considered a method for obtaining benzene homologues. Aluminum chloride is used as a catalyst, which promotes the decomposition of the haloalkane molecule into ions. It also needs heating.
Fourth property - alkylation of benzene with alkenes.
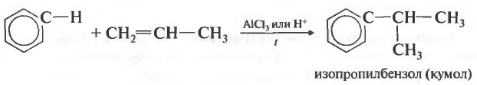
In this way, for example, cumene or ethylbenzene can be obtained. The catalyst is aluminum chloride.
2. Reactions of addition to benzene
The second group of reactions is addition reactions. We said that these reactions are not characteristic, but they are possible under rather harsh conditions with the destruction of the pi-electron cloud and the formation of six sigma bonds.
Fifth property in the general list - hydrogenation, addition of hydrogen.
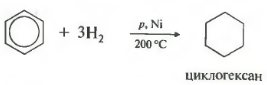
Temperature, pressure, catalyst nickel or platinum. Toluene is able to react in the same way.
sixth property - chlorination. Please note that we are talking specifically about the interaction with chlorine, since bromine does not enter into this reaction.
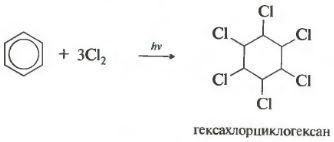
The reaction proceeds under hard ultraviolet irradiation. Hexachlorocyclohexane, another name for hexachlorane, is formed, a solid.
It is important to remember that for benzene not possible addition reactions of hydrogen halides (hydrohalogenation) and addition of water (hydration).
3. Substitution in the side chain of benzene homologues
The third group of reactions concerns only benzene homologues - this is a substitution in the side chain.
seventh a property in the general list is halogenation at the alpha carbon atom in the side chain.

The reaction occurs when heated or irradiated, and always only at the alpha carbon. As the halogenation continues, the second halogen atom will return to the alpha position.
4. Oxidation of benzene homologues
The fourth group of reactions is oxidation.
The benzene ring is too strong, so benzene does not oxidize potassium permanganate - does not discolor its solution. This is very important to remember.
On the other hand, benzene homologues are oxidized with an acidified solution of potassium permanganate when heated. And this is the eighth chemical property.

It turns out benzoic acid. Discoloration of the solution is observed. In this case, no matter how long the carbon chain of the substituent is, it always breaks after the first carbon atom and the alpha atom is oxidized to a carboxyl group with the formation of benzoic acid. The rest of the molecule is oxidized to the corresponding acid or, if it is only one carbon atom, to carbon dioxide.
If the benzene homologue has more than one hydrocarbon substituent on the aromatic ring, then the oxidation occurs according to the same rules - the carbon in the alpha position is oxidized.

In this example, a dibasic aromatic acid is obtained, which is called phthalic acid.
In a special way, I note the oxidation of cumene, isopropylbenzene, with atmospheric oxygen in the presence of sulfuric acid.

This is the so-called cumene method for producing phenol. As a rule, one has to deal with this reaction in matters relating to the production of phenol. This is the industrial way.
ninth property - combustion, complete oxidation with oxygen. Benzene and its homologues burn to carbon dioxide and water.
Let us write the equation for the combustion of benzene in a general form.
According to the law of conservation of mass, there should be as many atoms on the left as there are atoms on the right. Because, after all, in chemical reactions, atoms do not go anywhere, but the order of bonds between them simply changes. So there will be as many carbon dioxide molecules as there are carbon atoms in an arene molecule, since the molecule contains one carbon atom. That is n CO 2 molecules. There will be half as many water molecules as hydrogen atoms, that is, (2n-6) / 2, which means n-3.
There are the same number of oxygen atoms on the left and on the right. On the right, there are 2n from carbon dioxide, because there are two oxygen atoms in each molecule, plus n-3 from water, for a total of 3n-3. On the left, there are the same number of oxygen atoms - 3n-3, which means there are half as many molecules, because the molecule contains two atoms. That is (3n-3)/2 oxygen molecules.
Thus, we have compiled the equation for the combustion of benzene homologues in a general form.
what does benzene interact with and their reaction equations
- most characteristic of them are the substitution reactions of the hydrogen atoms of the benzene ring. They flow more easily than saturated hydrocarbons. Many organic compounds are obtained in this way. So, in the interaction of benzene with bromine (in the presence of a FeBr2 catalyst), a hydrogen atom is replaced by a bromine atom:
With another catalyst, all hydrogen atoms in benzene can be replaced by halogen. This happens, for example, when chlorine is passed into benzene in the presence of aluminum chloride:
Hexachlorobenzene is a colorless crystalline substance used for seed dressing and wood preservation.
If benzene is treated with a mixture of concentrated nitric and sulfuric acids (nitrating mixture), then the hydrogen atom is replaced by the NO2 nitro group:
In a benzene molecule, a hydrogen atom can be replaced by an alkyl radical by the action of halogen derivatives of hydrocarbons in the presence of aluminum chloride:
Addition reactions to benzene proceed with great difficulty. For their occurrence, special conditions are necessary: an increase in temperature and pressure, the selection of a catalyst, light irradiation, etc. So, in the presence of a catalyst - nickel or platinum - benzene is hydrogenated, i.e., it adds hydrogen, forming cyclohexane:
Under ultraviolet irradiation, benzene adds chlorine:
Hexachlorocyclohexane, or hexachlorane, is a crystalline substance used as a powerful insecticide.
Benzene does not add hydrogen halides and water. It is very resistant to oxidants. Unlike unsaturated hydrocarbons, it does not discolor bromine water and KMnO4 solution. Under normal conditions, the benzene ring is not destroyed by the action of many other oxidizing agents. However, benzene homologues undergo oxidation more easily than saturated hydrocarbons. In this case, only the radicals associated with the benzene ring undergo oxidation:
Thus, aromatic hydrocarbons can enter into both substitution and addition reactions, however, the conditions for these transformations differ significantly from similar transformations of saturated and unsaturated hydrocarbons.
Receipt. Benzene and its homologues are obtained in large quantities from petroleum and coal tar formed during the dry distillation of coal (coking). Dry distillation is carried out at coke and gas plants.
The reaction of converting cyclohexane to benzene (dehydrogenation or dehydrogenation) proceeds by passing it over a catalyst (platinum black) at 300C. Saturated hydrocarbons can also be converted into aromatics by the dehydrogenation reaction. For example:
Dehydrogenation reactions make it possible to use oil hydrocarbons to produce hydrocarbons of the benzene series. They indicate the relationship between different groups of hydrocarbons and their mutual transformation into each other.
According to the method of N. D. Zelinsky and B. A. Kazansky, benzene can be obtained by passing acetylene through a tube with activated carbon heated to 600 ° C. The whole process of polymerization of three molecules of acetylene can be represented by the diagram
- 1) substitution reaction
a) in the presence of a catalyst-salts of iron (III) - benzene enters into a substitution reaction:
C6H6+Br2=C6H5Br+Rick
benzene reacts similarly with chlorine
b) substitution reactions also include the interaction of benzene with nitric acid:
C6H6+HONO2=C6H5NO2+H2O
2) REACTION OF ADDITION
A) in action sunlight whether ultraviolet rays, benzene enters into an addition reaction. For example, when exposed to light, benzene adds chromium to form hexachlorocyclohexane:
C6H6+3Cl2=C6H6Cl6
b) benzene can also be hydrogenated:
C6HC+3H2=C6H12
3) OXIDATION REACTIONS
a) under the action of energetic oxidizing agents (KMnO4) on benzene homologues, only side chains undergo oxidation.
C6H5-CH3+3O=C7H6O2+H2O
b) benzene and its homologues burn with a flame in air:
2C6H6+15O2=12CO2+6H2O
DEFINITION
Benzene(cyclohexatriene - 1,3,5) - organic matter, the simplest representative of a number of aromatic hydrocarbons.
Formula - C 6 H 6 ( structural formula- rice. one). Molecular weight - 78, 11.
Rice. 1. Structural and spatial formulas of benzene.
All six carbon atoms in the benzene molecule are in the sp 2 hybrid state. Each carbon atom forms 3σ bonds with two other carbon atoms and one hydrogen atom lying in the same plane. Six carbon atoms form a regular hexagon (σ-skeleton of the benzene molecule). Each carbon atom has one unhybridized p-orbital, which contains one electron. Six p-electrons form a single π-electron cloud (aromatic system), which is depicted as a circle inside a six-membered cycle. The hydrocarbon radical derived from benzene is called C 6 H 5 - - phenyl (Ph-).
Chemical properties of benzene
Benzene is characterized by substitution reactions proceeding according to the electrophilic mechanism:
- halogenation (benzene interacts with chlorine and bromine in the presence of catalysts - anhydrous AlCl 3, FeCl 3, AlBr 3)
C 6 H 6 + Cl 2 \u003d C 6 H 5 -Cl + HCl;
- nitration (benzene easily reacts with a nitrating mixture - a mixture of concentrated nitric and sulfuric acids)

- alkylation with alkenes
C 6 H 6 + CH 2 \u003d CH-CH 3 → C 6 H 5 -CH (CH 3) 2;
Addition reactions to benzene lead to the destruction of the aromatic system and proceed only under harsh conditions:
- hydrogenation (the reaction proceeds when heated, the catalyst is Pt)

- addition of chlorine (occurs under the action of UV radiation with the formation of a solid product - hexachlorocyclohexane (hexachlorane) - C 6 H 6 Cl 6)

Like any organic compound, benzene enters into a combustion reaction with the formation of carbon dioxide and water as reaction products (it burns with a smoky flame):
2C 6 H 6 + 15O 2 → 12CO 2 + 6H 2 O.
Physical properties of benzene
Benzene is a colorless liquid, but has a specific pungent odor. Forms an azeotropic mixture with water, mixes well with ethers, gasoline and various organic solvents. Boiling point - 80.1C, melting point - 5.5C. Toxic, carcinogen (i.e. contributes to the development of cancer).
Obtaining and using benzene
The main methods for obtaining benzene:
— dehydrocyclization of hexane (catalysts - Pt, Cr 3 O 2)
CH 3 -(CH 2) 4 -CH 3 → C 6 H 6 + 4H 2;
- dehydrogenation of cyclohexane (the reaction proceeds when heated, the catalyst is Pt)
C 6 H 12 → C 6 H 6 + 4H 2;
– trimerization of acetylene (the reaction proceeds when heated to 600C, the catalyst is activated carbon)
3HC≡CH → C 6 H 6 .
Benzene serves as a raw material for the production of homologues (ethylbenzene, cumene), cyclohexane, nitrobenzene, chlorobenzene, and other substances. Previously, benzene was used as an additive to gasoline to increase its octane number, however, now, due to its high toxicity, the content of benzene in fuel is strictly regulated. Sometimes benzene is used as a solvent.
Examples of problem solving
EXAMPLE 1
| Exercise | Write down the equations with which you can carry out the following transformations: CH 4 → C 2 H 2 → C 6 H 6 → C 6 H 5 Cl. |
| Solution | To obtain acetylene from methane, the following reaction is used: 2CH 4 → C 2 H 2 + 3H 2 (t = 1400C). Obtaining benzene from acetylene is possible by the reaction of trimerization of acetylene, which occurs when heated (t = 600C) and in the presence of activated carbon: 3C 2 H 2 → C 6 H 6 . The chlorination reaction of benzene to obtain chlorobenzene as a product is carried out in the presence of iron (III) chloride: C 6 H 6 + Cl 2 → C 6 H 5 Cl + HCl. |
EXAMPLE 2
| Exercise | To 39 g of benzene in the presence of iron (III) chloride was added 1 mol of bromine water. What amount of the substance and how many grams of what products did this result in? |
| Solution | Let us write the equation for the reaction of benzene bromination in the presence of iron (III) chloride: C 6 H 6 + Br 2 → C 6 H 5 Br + HBr. The reaction products are bromobenzene and hydrogen bromide. Molar mass benzene calculated using the table chemical elements DI. Mendeleev - 78 g/mol. Find the amount of benzene substance: n(C 6 H 6) = m(C 6 H 6) / M(C 6 H 6); n(C 6 H 6) = 39/78 = 0.5 mol. According to the condition of the problem, benzene reacted with 1 mol of bromine. Consequently, benzene is in short supply and further calculations will be made for benzene. According to the reaction equation n (C 6 H 6): n (C 6 H 5 Br) : n (HBr) \u003d 1: 1: 1, therefore n (C 6 H 6) \u003d n (C 6 H 5 Br) \u003d: n(HBr) = 0.5 mol. Then, the masses of bromobenzene and hydrogen bromide will be equal: m(C 6 H 5 Br) = n(C 6 H 5 Br)×M(C 6 H 5 Br); m(HBr) = n(HBr)×M(HBr). Molar masses of bromobenzene and hydrogen bromide, calculated using the table of chemical elements of D.I. Mendeleev - 157 and 81 g/mol, respectively. m(C 6 H 5 Br) = 0.5×157 = 78.5 g; m(HBr) = 0.5 x 81 = 40.5 g. |
| Answer | The reaction products are bromobenzene and hydrogen bromide. The masses of bromobenzene and hydrogen bromide are 78.5 and 40.5 g, respectively. |
The cyclic structure of benzene was first proposed by F.A. Kekule in 1865

Friedrich August Kekule von Stradonitz was an outstanding German chemist of the 19th century. In 1854, he discovered the first organic compound containing sulfur - thioacetic acid (thioethanoic acid). In addition, he established the structure of diazo compounds. However, his most famous contribution to the development of chemistry is the establishment of the structure of benzene (1866). Kekule showed that the double bonds of benzene alternate around the ring (this idea first occurred to him in a dream). He later showed that the two possible double bond arrangements are identical and that the benzene ring is a hybrid between the two structures. Thus, he anticipated the idea of resonance (mesomerism), which appeared in the theory chemical bond in the early 1930s.
If benzene really had such a structure, then its 1,2-disubstituted derivatives should have two isomers each. For example,

However, none of the 1,2-disubstituted benzenes can isolate two isomers.
Therefore, Kekule subsequently suggested that the benzene molecule exists as two structures rapidly passing into each other:

Note that such schematic representations of benzene molecules and their derivatives usually do not indicate the hydrogen atoms attached to the carbon atoms of the benzene ring.
In modern chemistry, the benzene molecule is considered as a resonant hybrid of these two limiting resonant forms (see Section 2.1). Another description of the benzene molecule is based on a consideration of its molecular orbitals. In sec. 3.1, it was indicated that the -electrons located in the -bonding orbitals are delocalized between all carbon atoms of the benzene ring and form an -electron cloud. In accordance with this representation, the benzene molecule can be conventionally depicted as follows:
Experimental data confirm the presence of just such a structure in benzene. If benzene had the structure that Kekule originally proposed, with three conjugated double bonds, then benzene would have to enter into addition reactions like alkenes. However, as mentioned above, benzene does not enter into addition reactions. In addition, benzene is more stable than if it had three isolated double bonds. In sec. 5.3 it was indicated that the enthalpy of hydrogenation of benzene with the formation of cyclohexane has a larger negative
Table 18.3. Length of various carbon-carbon bonds


Rice. 18.6. The geometric structure of the benzene molecule.
value than three times the enthalpy of hydrogenation of cyclohexene. The difference between these values is usually called the delocalization enthalpy, resonant energy, or benzene stabilization energy.
All carbon-carbon bonds in the benzene ring have the same length, which is less than the length C-C connections in alkanes, but more than the length of the C \u003d C bonds in alkenes (Table 18.3). This confirms that the carbon-carbon bonds in benzene are a hybrid between single and double bonds.
The benzene molecule has a flat structure, which is shown in Fig. 18.6.
Physical Properties
Under normal conditions, benzene is a colorless liquid that freezes at 5.5°C and boils at 80°C. It has a characteristic pleasant smell, but, as mentioned above, is highly toxic. Benzene is immiscible with water, and in the benzene system, water forms the top of the two layers. However, it is soluble in non-polar organic solvents and is itself a good solvent for other organic compounds.
Chemical properties
Although benzene enters into certain addition reactions (see below), it does not exhibit the reactivity typical of alkenes in them. For example, it does not decolorize bromine water or α-ion solutions. In addition, benzene
enters into addition reactions with strong acids, such as hydrochloric or sulfuric acid.
At the same time, benzene takes part in a number of electrophilic substitution reactions. Aromatic compounds are the products of reactions of this type, since the delocalized -electron system of benzene is preserved in these reactions. The general mechanism of substitution of a hydrogen atom on a benzene ring by some electrophile is described in Sec. 17.3. Examples of electrophilic substitution of benzene are its nitration, halogenation, sulfonation, and Friedel-Crafts reactions.
Nitration. Benzene can be nitrated (introducing a group into it) by treating it with a mixture of concentrated nitric and sulfuric acids:

Nitrobenzene
The conditions for this reaction and its mechanism are described in Sec. 17.3.
Nitrobenzene is a pale yellow liquid with a characteristic almond odor. During the nitration of benzene, in addition to nitrobenzene, crystals of 1,3-dinitrobenzene are also formed, which is the product of the following reaction:

Halogenation. If you mix benzene in the dark with chlorine or bromine, no cancer will occur. However, in the presence of catalysts with the properties of Lewis acids, electrophilic substitution reactions occur in such mixtures. Typical catalysts for these reactions are iron(III) bromide and aluminum chloride. The action of these catalysts is that they create polarization in the halogen molecules, which then form a complex with the catalyst:
although there is no direct evidence that free ions are formed in this case. The mechanism of benzene bromination using iron (III) bromide as an ion carrier can be represented as follows:

Sulfonation. Benzene can be sulfonated (replacing a hydrogen atom in it with a sulfo group) by refluxing its mixture with concentrated sulfuric acid for several hours. Instead, benzene can be gently heated mixed with fuming sulfuric acid. Fuming sulfuric acid contains sulfur trioxide. The mechanism of this reaction can be represented by the scheme

Friedel-Crafts reactions. Friedel-Crafts reactions were originally called condensation reactions between aromatic compounds and alkyl halides in the presence of an anhydrous aluminum chloride catalyst.
In condensation reactions, two molecules of reactants (or one reactant) are combined with each other, forming a molecule of a new compound, while a molecule of some simple compound, such as water or hydrogen chloride, is split off (eliminates) from them.
Currently, the Friedel-Crafts reaction is any electrophilic substitution of an aromatic compound in which a carbocation or a highly polarized complex with a positively charged carbon atom plays the role of an electrophile. The electrophilic agent is usually an alkyl halide or some kind of chloride. carboxylic acid, although in the same way it can be, for example, an alkene or an alcohol. Anhydrous aluminum chloride is usually used as a catalyst for these reactions. Friedel-Crafts reactions are usually divided into two types: alkylation and acylation.
Alkylation. In Friedel-Crafts reactions of this type, one or more hydrogen atoms in the benzene ring are replaced by alkyl groups. For example, when a mixture of benzene and chloromethane is heated carefully in the presence of anhydrous aluminum chloride, methylbenzene is formed. Chloromethane plays the role of an electrophilic agent in this reaction. It is polarized by aluminum chloride in the same way as it happens with halogen molecules:
The mechanism of the considered reaction can be represented as follows:

It should be noted that in this condensation reaction between benzene and chloromethane, a hydrogen chloride molecule is split off. We also note that the real existence of a metal carbocation in the form of a free ion is doubtful.
Alkylation of benzene with chloromethane in the presence of anhydrous aluminum chloride catalyst does not end with the formation of methylbenzene. In this reaction, further alkylation of the benzene ring occurs, leading to the formation of 1,2-dimethylbenzene:

Acylation. In Friedel-Crafts reactions of this type, the hydrogen atom in the benzene ring is replaced by an acyl group, resulting in the formation of an aromatic ketone.
The acyl group has the general formula
The systematic name of an acyl compound is formed by replacing the suffix and ending -ova in the name of the corresponding carboxylic acid, of which the given acyl compound is a derivative, with the suffix -(o)yl. For example

Acylation of benzene is carried out using a chloride or anhydride of a carboxylic acid in the presence of an anhydrous aluminum chloride catalyst. For example

This reaction is a condensation in which the elimination of a hydrogen chloride molecule occurs. Note also that the name "phenyl" is often used to denote the benzene ring in compounds where benzene is not the main group:

Addition reactions. Although benzene is most characteristic of electrophilic substitution reactions, it also enters into some addition reactions. We have already met one of them. It's about on the hydrogenation of benzene (see Section 5.3). When a mixture of benzene and hydrogen is passed over the surface of a finely ground nickel catalyst at a temperature of 150–160 °C, a whole sequence of reactions occurs, which ends with the formation of cyclohexane. The overall stoichiometric equation for this reaction can be represented as follows:

Under the action of ultraviolet radiation or direct sunlight, benzene also reacts with chlorine. This reaction is carried out by a complex radical mechanism. Its final product is 1,2,3,4,5,6-hexachlorocyclohexane:

A similar reaction occurs between benzene and bromine under the action of ultraviolet radiation or sunlight.
Oxidation. Benzene and the benzene ring in other aromatic compounds are generally resistant to oxidation even by such strong oxidizing agents as an acidic or alkaline solution of potassium permanganate. However, benzene and other aromatic compounds burn in air or oxygen to form a very smoky flame, which is typical for hydrocarbons with a high relative carbon content.



