Lesson 28
In Lesson 28 " Chemical properties of water» from the course « Chemistry for dummies» learn about the interaction of water with various substances.
Under normal conditions, water is a fairly active substance in relation to other substances. This means that it enters into chemical reactions with many of them.
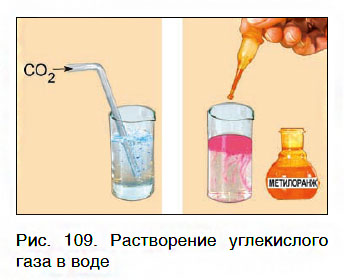
If a jet of gaseous carbon monoxide (IV) CO 2 (carbon dioxide) is directed into water, then part of it will dissolve in it (Fig. 109).
At the same time, a chemical reaction of the compound occurs in the solution, as a result of which a new substance is formed - carbonic acid H 2 CO 3:
![]()
On a note: Collecting carbon dioxide over water, J. Priestley discovered that part of the gas dissolves in water and gives it a pleasant tart taste. In fact, Priestley was the first to get a drink like soda, or soda.wow, water.
The compound reaction also occurs if a solid is added to water. phosphorus(V) oxide P 2 O 5. In this case, a chemical reaction takes place with the formation phosphoric acid H 3 PO 4(Fig. 110):

Let's test the solutions obtained by the interaction of CO 2 and P 2 O 5 with water, the indicator is methyl orange. To do this, add 1-2 drops of the indicator solution to the resulting solutions. The indicator color will change from orange to red what says about the presence acids in solutions. This means that during the interaction of CO 2 and P 2 O 5 with water, the acids H 2 CO 3 and H 3 PO 4 were indeed formed.
Oxides like CO 2 and P 2 O 5 , which form acids when interacting with water, are classified as acid oxides.
Acid oxides are oxides to which acids correspond.
Some of the acid oxides and their corresponding acids are listed in Table 11. Note that these are oxides of non-metal elements. Generally, non-metal oxides are acidic oxides.
Interaction with metal oxides
Water reacts differently with metal oxides than with non-metal oxides.
We study the interaction of calcium oxide CaO with water. To do this, place a small amount of CaO in a glass of water and mix thoroughly. In this case, a chemical reaction takes place:
as a result of which a new substance Ca (OH) 2 is formed, belonging to the class of bases. In the same way, oxides of lithium and sodium react with water. At the same time, bases are also formed, for example:
You will learn more about the bases in the next lesson. Metal oxides that correspond to bases are called basic oxides.
Basic oxides are oxides that correspond to bases.
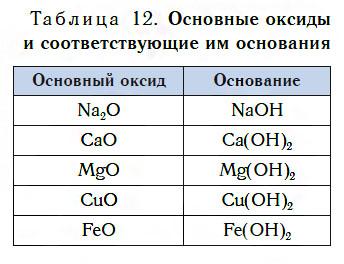
Table 12 lists the formulas for some of the basic oxides and their corresponding bases. Note that, unlike acidic oxides, basic oxides contain metal atoms. Most metal oxides are basic oxides.
Although each basic oxide has a corresponding base, not all basic oxides react with water like CaO to form bases.
Interaction with metals
Under normal conditions, active metals (K, Na, Ca, Ba, etc.) react violently with water:
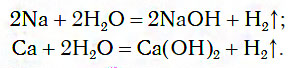
These reactions release hydrogen and form water-soluble bases.
As a chemically active substance, water reacts with many other substances, but you will learn about this when you study chemistry further.
Lesson summary:
- Water is a chemically active substance. It reacts with acidic and basic oxides, active metals.
- When water reacts with most acidic oxides, the corresponding acids are formed.
- Some basic oxides react with water to form soluble bases.
- Under normal conditions, water reacts with the most active metals. This produces soluble bases and hydrogen.
I hope lesson 28 " Chemical properties of water' was clear and informative. If you have any questions, write them in the comments.