Topic 10. Covalent polar chemical bond.
Part I
1. Electronegativity (EO) is the ability of atoms to attract electron pairs to themselves.
3. If a covalent chemical bond is formed between atoms of different non-metal elements, then common electron pairs are biased towards the more electronegative element. An excess negative charge arises on it, and an excess positive charge arises on the partner atom. Such a connection is called covalent polar.
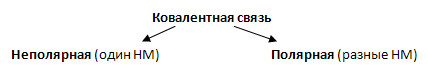

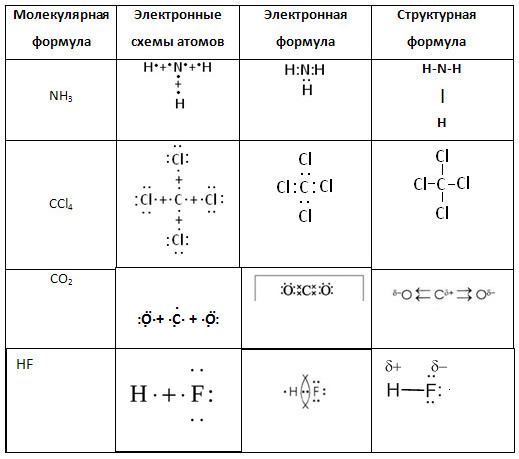
5. Complete the table "Covalent polar bond".
Part II
1. Play tic-tac-toe. Show the winning path, consisting of formulas of substances with a covalent polar bond, and write down the schemes for their formation.
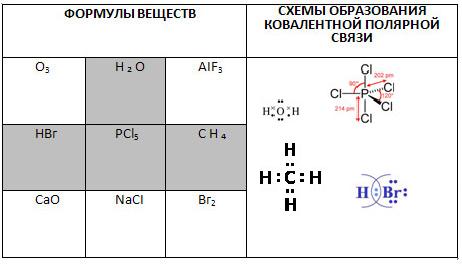
2. Select the formulas of compounds with a covalent polar chemical bond. From the letters corresponding to the correct answers, you will form a word meaning an imitation of a diamond or other precious stone made of glass: rhinestone.
1) HF C
3) FeBr3 T
5) SO2 P
7) CO2 A
9) PCl5 Z
3. Plot the dependence of the serial number of a chemical element on the electronegativity of elements of the same period. Find the exact values of electronegativity using the Internet. Conclude:
With an increase in the serial number, the EO grows.
4. Plot the dependence of the serial number of a chemical element on the electronegativity of the elements of one main subgroup. Find the exact values of electronegativity using the Internet.
In the group with an increase in the serial number, EO decreases.
5. The most polar is the chemical bond in the molecule:
4) hydrogen fluoride - HF
6. Arrange the following substances in order of decreasing polarity of the chemical bond.
4) potassium phosphide - K3P
2) aluminum phosphide - AlP
3) phosphorus (V) chloride - PCl5
1) white phosphorus - P4