Periodic table complete in good quality
The periodic table is one of the greatest discoveries of mankind, which made it possible to streamline knowledge about the world around us and discover new chemical elements. It is necessary for schoolchildren, as well as for everyone who is interested in chemistry. In addition, this scheme is indispensable in other areas of science.
This scheme contains all the elements known to man, and they are grouped depending on atomic mass and serial number. These characteristics affect the properties of the elements. In total, there are 8 groups in the short version of the table, the elements included in one group have very similar properties. The first group contains hydrogen, lithium, potassium, copper, the Latin pronunciation in Russian of which is cuprum. And also argentum - silver, cesium, gold - aurum and francium. The second group contains beryllium, magnesium, calcium, zinc, followed by strontium, cadmium, barium, and the group ends with mercury and radium.
The third group includes boron, aluminum, scandium, gallium, then yttrium, indium, lanthanum, and the group ends with thallium and actinium. The fourth group begins with carbon, silicon, titanium, continues with germanium, zirconium, tin, and ends with hafnium, lead, and rutherfordium. In the fifth group there are elements such as nitrogen, phosphorus, vanadium, arsenic, niobium, antimony are located below, then bismuth tantalum comes and completes the dubnium group. The sixth begins with oxygen, followed by sulfur, chromium, selenium, then molybdenum, tellurium, then tungsten, polonium and seaborgium.
In the seventh group, the first element is fluorine, followed by chlorine, manganese, bromine, technetium, followed by iodine, then rhenium, astatine and borium. The last group is the most numerous. It includes gases such as helium, neon, argon, krypton, xenon and radon. This group also includes the metals iron, cobalt, nickel, rhodium, palladium, ruthenium, osmium, iridium, platinum. Next come hannium and meitnerium. Separately located elements that form the actinide series and the lanthanide series. They have similar properties to lanthanum and actinium.

This scheme includes all types of elements, which are divided into 2 large groups - metals and non-metals with different properties. How to determine whether an element belongs to a particular group, a conditional line will help, which must be drawn from boron to astatine. It should be remembered that such a line can only be drawn in the full version of the table. All elements that are above this line and are located in the main subgroups are considered non-metals. And which are lower, in the main subgroups - metals. Also, metals are substances that are in side subgroups. There are special pictures and photos on which you can get acquainted with the position of these elements in detail. It is worth noting that those elements that are on this line exhibit the same properties of both metals and non-metals.
A separate list is also made up of amphoteric elements, which have dual properties and can form 2 types of compounds as a result of reactions. At the same time, they manifest equally both basic and acid properties. The predominance of certain properties depends on the reaction conditions and the substances with which the amphoteric element reacts.
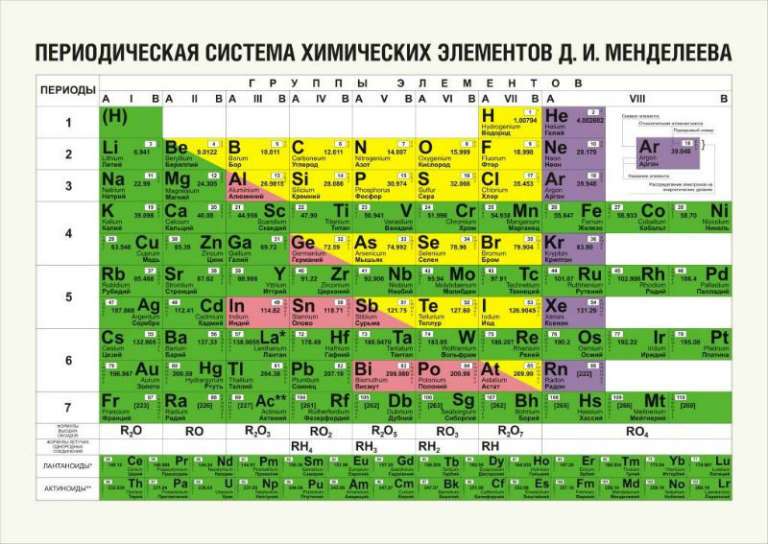
It should be noted that this scheme in the traditional execution of good quality is color. At the same time, different colors for ease of orientation are indicated main and secondary subgroups. And also elements are grouped depending on the similarity of their properties.
However, at present, along with the color scheme, the black-and-white periodic table of Mendeleev is very common. This form is used for black and white printing. Despite the apparent complexity, working with it is just as convenient, given some of the nuances. So, in this case, it is possible to distinguish the main subgroup from the secondary one by differences in shades that are clearly visible. In addition, in the color version, elements with the presence of electrons on different layers are indicated different colors.
It is worth noting that in a single-color design it is not very difficult to navigate the scheme. For this, the information indicated in each individual cell of the element will be enough.
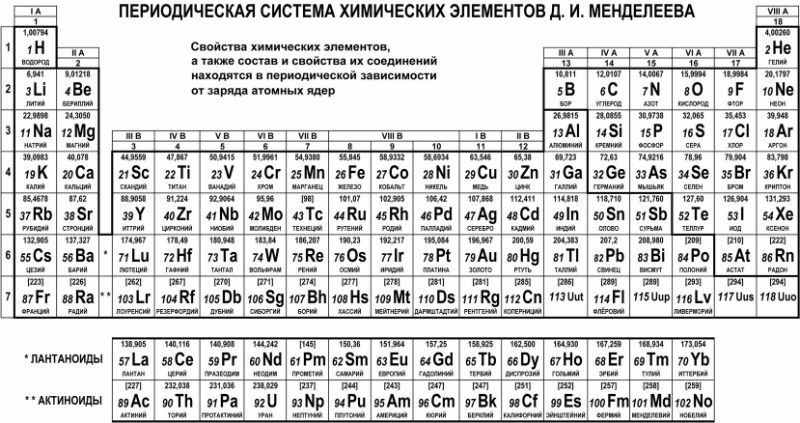
The exam today is the main type of test at the end of school, which means that special attention must be paid to preparing for it. Therefore, when choosing final exam in chemistry, you need to pay attention to the materials that can help in its delivery. As a rule, schoolchildren are allowed to use some tables during the exam, in particular, the periodic table in good quality. Therefore, in order for it to bring only benefit in tests, attention should be paid in advance to its structure and the study of the properties of the elements, as well as their sequence. You also need to learn use the black and white version of the table so that you don't face any difficulties in the exam.

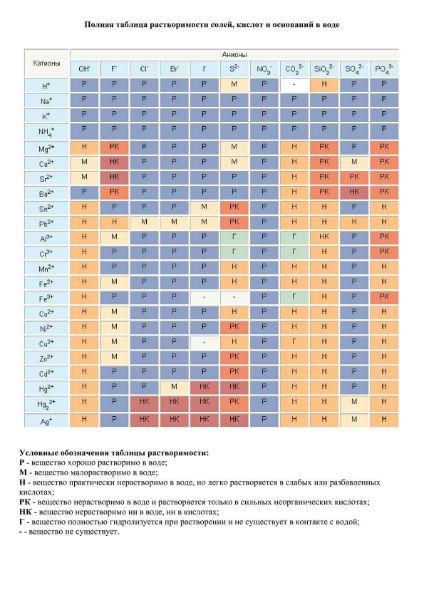
In addition to the main table characterizing the properties of elements and their dependence on atomic mass, there are other schemes that can help in the study of chemistry. For example, there are tables of solubility and electronegativity of substances. The first one can determine how soluble a particular compound is in water at ordinary temperature. In this case, anions are located horizontally - negatively charged ions, and cations, that is, positively charged ions, are located vertically. To find out degree of solubility of one or another compound, it is necessary to find its components in the table. And at the place of their intersection there will be the necessary designation.
If it is the letter "r", then the substance is completely soluble in water under normal conditions. In the presence of the letter "m" - the substance is slightly soluble, and in the presence of the letter "n" - it almost does not dissolve. If there is a “+” sign, the compound does not form a precipitate and reacts with the solvent without residue. If a "-" sign is present, it means that such a substance does not exist. Sometimes you can also see the sign “?” in the table, then this means that the degree of solubility of this compound is not known for certain. Electronegativity of the elements can vary from 1 to 8, there is also a special table to determine this parameter.
 Another useful table is the metal activity series. All metals are located in it by increasing the degree of electrochemical potential. A series of stress metals begins with lithium, ends with gold. It is believed that the more to the left a metal occupies in this row, the more active it is in chemical reactions. Thus, the most active metal Lithium is considered to be an alkaline metal. Hydrogen is also present at the end of the list of elements. It is believed that the metals that are located after it are practically inactive. Among them are elements such as copper, mercury, silver, platinum and gold.
Another useful table is the metal activity series. All metals are located in it by increasing the degree of electrochemical potential. A series of stress metals begins with lithium, ends with gold. It is believed that the more to the left a metal occupies in this row, the more active it is in chemical reactions. Thus, the most active metal Lithium is considered to be an alkaline metal. Hydrogen is also present at the end of the list of elements. It is believed that the metals that are located after it are practically inactive. Among them are elements such as copper, mercury, silver, platinum and gold.
Periodic table pictures in good quality
This scheme is one of the greatest achievements in the field of chemistry. Wherein There are many types of this table.- a short version, a long one, as well as an extra long one. The most common is the short table, and the long version of the schema is also common. It is worth noting that the short version of the scheme is not currently recommended by IUPAC for use.
Total was more than a hundred types of tables have been developed, which differ in presentation, shape, and graphical representation. They are used in various fields of science, or not used at all. Currently, new circuit configurations continue to be developed by researchers. As the main option, either a short or a long circuit in excellent quality is used.