Periodic law of D. I. Mendeleev and the periodic system of chemical elements
Periodic law D.I. Mendeleev and the Periodic Table of Chemical Elements is of great importance in the development of chemistry. Let's plunge into 1871, when professor of chemistry D.I. Mendeleev, through numerous trial and error, came to the conclusion that "... the properties of the elements, and therefore the properties of the simple and complex bodies they form, stand in a periodic dependence on their atomic weight." The periodicity of changes in the properties of elements arises due to the periodic repetition of the electronic configuration of the outer electronic layer with an increase in the charge of the nucleus.
Modern formulation of the periodic law is:
"the properties of chemical elements (i.e., the properties and form of the compounds they form) are in a periodic dependence on the charge of the nucleus of atoms of chemical elements."
While teaching chemistry, Mendeleev understood that remembering the individual properties of each element causes difficulties for students. He began to look for ways to create a system method to make it easier to remember the properties of elements. As a result, there was natural table, later it became known as periodical.
Our modern table is very similar to Mendeleev's. Let's consider it in more detail.
periodic table
The periodic table of Mendeleev consists of 8 groups and 7 periods.
The vertical columns of a table are called groups . The elements within each group have similar chemical and physical properties. This is explained by the fact that the elements of one group have similar electronic configurations of the outer layer, the number of electrons on which is equal to the group number. The group is then divided into main and secondary subgroups.
AT Main subgroups includes elements whose valence electrons are located on the outer ns- and np-sublevels. AT Side subgroups includes elements whose valence electrons are located on the outer ns-sublevel and the inner (n - 1) d-sublevel (or (n - 2) f-sublevel).
All elements in periodic table , depending on which sublevel (s-, p-, d- or f-) are valence electrons are classified into: s-elements (elements of the main subgroups I and II groups), p-elements (elements of the main subgroups III - VII groups), d- elements (elements of side subgroups), f- elements (lanthanides, actinides).
The highest valence of an element (with the exception of O, F, elements of the copper subgroup and the eighth group) is equal to the number of the group in which it is located.
For elements of the main and secondary subgroups, the formulas of higher oxides (and their hydrates) are the same. In the main subgroups, the composition of hydrogen compounds is the same for the elements in this group. Solid hydrides form elements of the main subgroups of groups I-III, and groups IV-VII form gaseous hydrogen compounds. Hydrogen compounds of the EN 4 type are more neutral compounds, EN 3 are bases, H 2 E and NE are acids.
The horizontal rows of the table are called periods. Elements in periods differ from each other, but they have in common that the last electrons are at the same energy level ( principal quantum numbern- equally ).
The first period differs from the others in that there are only 2 elements there: hydrogen H and helium He.
There are 8 elements (Li - Ne) in the second period. Lithium Li - an alkali metal begins the period, and closes its noble gas neon Ne.
In the third period, as well as in the second, there are 8 elements (Na - Ar). The alkali metal sodium Na begins the period, and the noble gas argon Ar closes it.
In the fourth period there are 18 elements (K - Kr) - Mendeleev designated it as the first large period. It also begins with the alkali metal Potassium and ends with the inert gas krypton Kr. The composition of large periods includes transition elements (Sc - Zn) - d- elements.
In the fifth period, similarly to the fourth, there are 18 elements (Rb - Xe) and its structure is similar to the fourth. It also begins with the alkali metal rubidium Rb, and ends with the inert gas xenon Xe. The composition of large periods includes transition elements (Y - Cd) - d- elements.
The sixth period consists of 32 elements (Cs - Rn). Except 10 d-elements (La, Hf - Hg) it contains a row of 14 f-elements (lanthanides) - Ce - Lu
The seventh period is not over. It starts with Francium Fr, it can be assumed that it will contain, like the sixth period, 32 elements that have already been found (up to the element with Z = 118).
Interactive periodic table
If you look at Mendeleev's periodic table and draw an imaginary line starting at boron and ending between polonium and astatine, then all metals will be to the left of the line, and non-metals to the right. Elements immediately adjacent to this line will have the properties of both metals and non-metals. They are called metalloids or semimetals. These are boron, silicon, germanium, arsenic, antimony, tellurium and polonium.
Periodic Law
Mendeleev gave the following formulation of the Periodic Law: "the properties of simple bodies, as well as the forms and properties of the compounds of elements, and therefore the properties of the simple and complex bodies formed by them, stand in a periodic dependence on their atomic weight."
There are four main periodic patterns:
Octet rule states that all elements tend to gain or lose an electron in order to have the eight-electron configuration of the nearest noble gas. Because Since the outer s and p orbitals of the noble gases are completely filled, they are the most stable elements.
Ionization energy is the amount of energy required to detach an electron from an atom. According to the octet rule, moving from left to right across the periodic table requires more energy to detach an electron. Therefore, the elements on the left side of the table tend to lose an electron, and those on the right side - to gain it. Inert gases have the highest ionization energy. The ionization energy decreases as you move down the group, because electrons at low energy levels have the ability to repel electrons from higher energy levels. This phenomenon is called shielding effect. Due to this effect, the outer electrons are less strongly bound to the nucleus. Moving along the period, the ionization energy gradually increases from left to right.
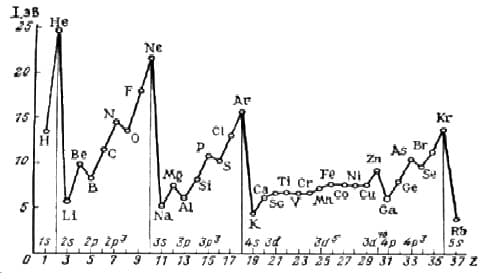
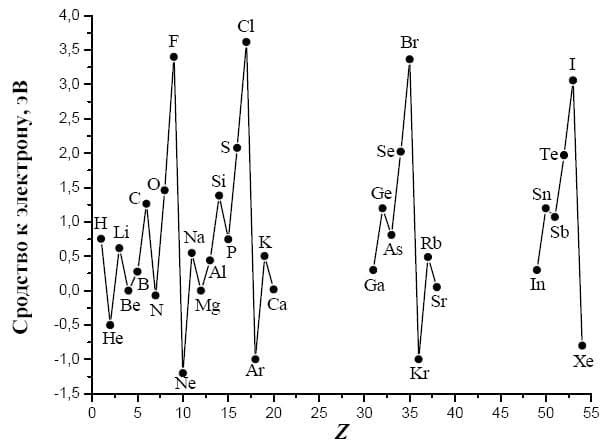
electron affinity is the change in energy upon acquisition of an additional electron by an atom of a substance in a gaseous state. When moving down the group, the electron affinity becomes less negative due to the screening effect.
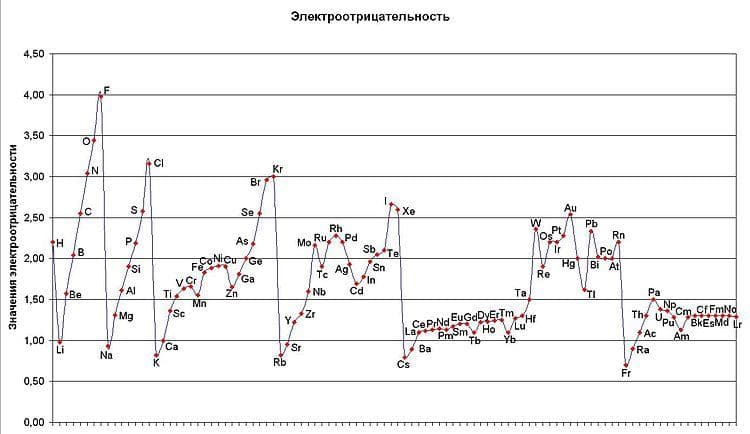
Electronegativity- a measure of how strongly it tends to attract the electrons of another atom bound to it. Electronegativity increases as you move periodic table left to right and bottom to top. It must be remembered that noble gases do not have electronegativity. Thus, the most electronegative element is fluorine.
Based on these concepts, let's consider how the properties of atoms and their compounds change in periodic table.
So, in a periodic dependence are such properties of an atom that are associated with its electronic configuration: atomic radius, ionization energy, electronegativity.
Consider the change in the properties of atoms and their compounds depending on the position in periodic table of chemical elements.
The non-metallicity of the atom increases when moving in the periodic table left to right and bottom to top. Concerning the basic properties of oxides decrease, and acid properties increase in the same order - from left to right and from bottom to top. At the same time, the acidic properties of oxides are the stronger, the greater the degree of oxidation of the element forming it
By period from left to right basic properties hydroxides weaken, in the main subgroups from top to bottom, the strength of the bases increases. At the same time, if a metal can form several hydroxides, then with an increase in the degree of oxidation of the metal, basic properties hydroxides weaken.
By period from left to right the strength of oxygen-containing acids increases. When moving from top to bottom within the same group, the strength of oxygen-containing acids decreases. In this case, the strength of the acid increases with an increase in the degree of oxidation of the acid-forming element.
By period from left to right the strength of anoxic acids increases. When moving from top to bottom within the same group, the strength of anoxic acids increases.
Categories ,