Periodic table of chemical elements of D.I. Mendeleev
In nature, there are a lot of repeating sequences:
- seasons;
- Times of Day;
- days of the week…
In the middle of the 19th century, D.I. Mendeleev noticed that the chemical properties of elements also have a certain sequence (they say that this idea came to him in a dream). The result of the miraculous dreams of the scientist was the Periodic Table of Chemical Elements, in which D.I. Mendeleev arranged the chemical elements in order of increasing atomic mass. In the modern table, the chemical elements are arranged in ascending order of the atomic number of the element (the number of protons in the nucleus of an atom).
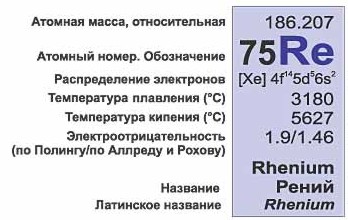
The atomic number is shown above the symbol of a chemical element, below the symbol is its atomic mass (the sum of protons and neutrons). Note that the atomic mass of some elements is a non-integer! Remember isotopes! Atomic mass is the weighted average of all the isotopes of an element that occur naturally under natural conditions.
Below the table are the lanthanides and actinides.
Metals, non-metals, metalloids
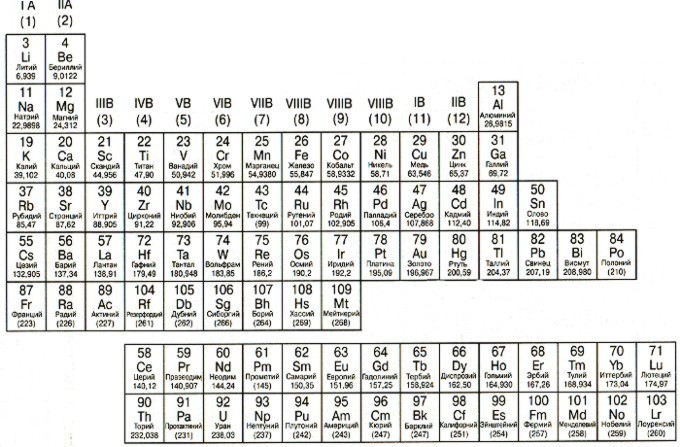
They are located in the Periodic Table to the left of the stepped diagonal line that starts with Boron (B) and ends with polonium (Po) (the exceptions are germanium (Ge) and antimony (Sb). It is easy to see that metals occupy most of the Periodic Table. The main properties of metals : solid (except mercury); shiny; good electrical and thermal conductors; ductile; malleable; easily donate electrons.
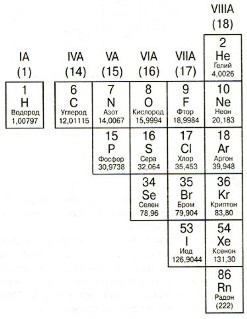
The elements to the right of the stepped diagonal B-Po are called non-metals. The properties of non-metals are directly opposite to the properties of metals: poor conductors of heat and electricity; fragile; non-forged; non-plastic; usually accept electrons.
Metalloids
Between metals and non-metals are semimetals(metalloids). They are characterized by the properties of both metals and non-metals. Semimetals have found their main industrial application in the production of semiconductors, without which no modern microcircuit or microprocessor is inconceivable.
Periods and groups
As mentioned above, the periodic table consists of seven periods. In each period, the atomic numbers of the elements increase from left to right.
The properties of elements in periods change sequentially: so sodium (Na) and magnesium (Mg), which are at the beginning of the third period, give up electrons (Na gives up one electron: 1s 2 2s 2 2p 6 3s 1; Mg gives up two electrons: 1s 2 2s 2 2p 6 3s 2). But chlorine (Cl), located at the end of the period, takes one element: 1s 2 2s 2 2p 6 3s 2 3p 5.
In groups, on the contrary, all elements have the same properties. For example, in the IA(1) group, all elements from lithium (Li) to francium (Fr) donate one electron. And all elements of group VIIA(17) take one element.
Some groups are so important that they have been given special names. These groups are discussed below.
Group IA(1). The atoms of the elements of this group have only one electron in the outer electron layer, so they easily donate one electron.
The most important alkali metals are sodium (Na) and potassium (K), since they play an important role in the process of human life and are part of salts.
Electronic configurations:
- Li- 1s 2 2s 1 ;
- Na- 1s 2 2s 2 2p 6 3s 1 ;
- K- 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1
Group IIA(2). The atoms of the elements of this group have two electrons in the outer electron layer, which also give up during chemical reactions. The most important element is calcium (Ca) - the basis of bones and teeth.
Electronic configurations:
- Be- 1s 2 2s 2 ;
- mg- 1s 2 2s 2 2p 6 3s 2 ;
- Ca- 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2
Group VIIA(17). Atoms of the elements of this group usually receive one electron each, because. on the outer electronic layer there are five elements each, and one electron is just missing to the "complete set".
The most famous elements of this group are: chlorine (Cl) - is part of salt and bleach; iodine (I) is an element that plays an important role in the activity of the human thyroid gland.
Electronic configuration:
- F- 1s 2 2s 2 2p 5 ;
- Cl- 1s 2 2s 2 2p 6 3s 2 3p 5 ;
- Br- 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5
Group VIII(18). Atoms of the elements of this group have a fully "staffed" outer electron layer. Therefore, they "do not need" to accept electrons. And they don't want to give them away. Hence - the elements of this group are very "reluctant" to enter into chemical reactions. For a long time it was believed that they do not react at all (hence the name "inert", i.e. "inactive"). But chemist Neil Barlett discovered that some of these gases, under certain conditions, can still react with other elements.
Electronic configurations:
- Ne- 1s 2 2s 2 2p 6 ;
- Ar- 1s 2 2s 2 2p 6 3s 2 3p 6 ;
- kr- 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6
Valence elements in groups
It is easy to see that within each group, the elements are similar to each other in their valence electrons (electrons of s and p orbitals located on the outer energy level).
Alkali metals have 1 valence electron each:
- Li- 1s 2 2s 1 ;
- Na- 1s 2 2s 2 2p 6 3s 1 ;
- K- 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1
Alkaline earth metals have 2 valence electrons:
- Be- 1s 2 2s 2 ;
- mg- 1s 2 2s 2 2p 6 3s 2 ;
- Ca- 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2
Halogens have 7 valence electrons:
- F- 1s 2 2s 2 2p 5 ;
- Cl- 1s 2 2s 2 2p 6 3s 2 3p 5 ;
- Br- 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5
Inert gases have 8 valence electrons:
- Ne- 1s 2 2s 2 2p 6 ;
- Ar- 1s 2 2s 2 2p 6 3s 2 3p 6 ;
- kr- 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6
For more information, see the article Valency and the Table of electronic configurations of atoms of chemical elements by periods.
Let us now turn our attention to the elements located in groups with symbols AT. They are located in the center of the periodic table and are called transition metals.
A distinctive feature of these elements is the presence of electrons in atoms that fill d-orbitals:
- sc- 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1 ;
- Ti- 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2
Separate from the main table are located lanthanides and actinides are the so-called internal transition metals. In the atoms of these elements, electrons fill f-orbitals:
- Ce- 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 4d 10 5s 2 5p 6 4f 1 5d 1 6s 2 ;
- Th- 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2 6p 6 6d 2 7s 2