General physical and chemical properties of metals
Due to the presence of free electrons (“electron gas”) in the crystal lattice, all metals exhibit the following characteristic general properties:
1) Plastic- the ability to easily change shape, stretch into a wire, roll into thin sheets.
2) metallic luster and opacity. This is due to the interaction of free electrons with light incident on the metal.
3) Electrical conductivity. It is explained by the directed movement of free electrons from the negative to the positive pole under the influence of a small potential difference. When heated, the electrical conductivity decreases, because. as the temperature rises, the vibrations of atoms and ions in the nodes of the crystal lattice increase, which makes it difficult for the directed movement of the “electron gas”.
4) Thermal conductivity. It is due to the high mobility of free electrons, due to which the temperature is quickly equalized by the mass of the metal. The highest thermal conductivity is in bismuth and mercury.
5) Hardness. The hardest is chrome (cuts glass); the softest - alkali metals - potassium, sodium, rubidium and cesium - are cut with a knife.
6) Density. It is the smaller, the smaller the atomic mass of the metal and the larger the radius of the atom. The lightest is lithium (ρ=0.53 g/cm3); the heaviest is osmium (ρ=22.6 g/cm3). Metals having a density less than 5 g/cm3 are considered "light metals".
7) Melting and boiling points. The most fusible metal is mercury (m.p. = -39°C), the most refractory metal is tungsten (t°m. = 3390°C). Metals with t°pl. above 1000°C are considered refractory, below - low melting point.
General chemical properties of metals
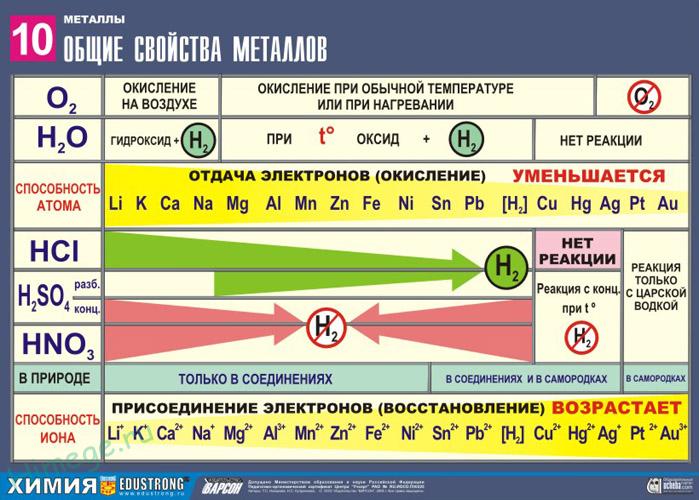
Strong reducing agents: Me 0 – nē → Me n +
A number of stresses characterize the comparative activity of metals in redox reactions in aqueous solutions.
1. Reactions of metals with non-metals
1) With oxygen:
2Mg + O 2 → 2MgO
2) With sulfur:
Hg + S → HgS
3) With halogens:
Ni + Cl 2 – t° → NiCl 2
4) With nitrogen:
3Ca + N 2 – t° → Ca 3 N 2
5) With phosphorus:
3Ca + 2P – t° → Ca 3 P 2
6) With hydrogen (only alkali and alkaline earth metals react):
2Li + H 2 → 2LiH
Ca + H 2 → CaH 2
2. Reactions of metals with acids
1) Metals standing in the electrochemical series of voltages up to H reduce non-oxidizing acids to hydrogen:
Mg + 2HCl → MgCl 2 + H 2
2Al+ 6HCl → 2AlCl 3 + 3H 2
6Na + 2H 3 PO 4 → 2Na 3 PO 4 + 3H 2
2) With oxidizing acids:
In the interaction of nitric acid of any concentration and concentrated sulfuric acid with metals hydrogen is never released!
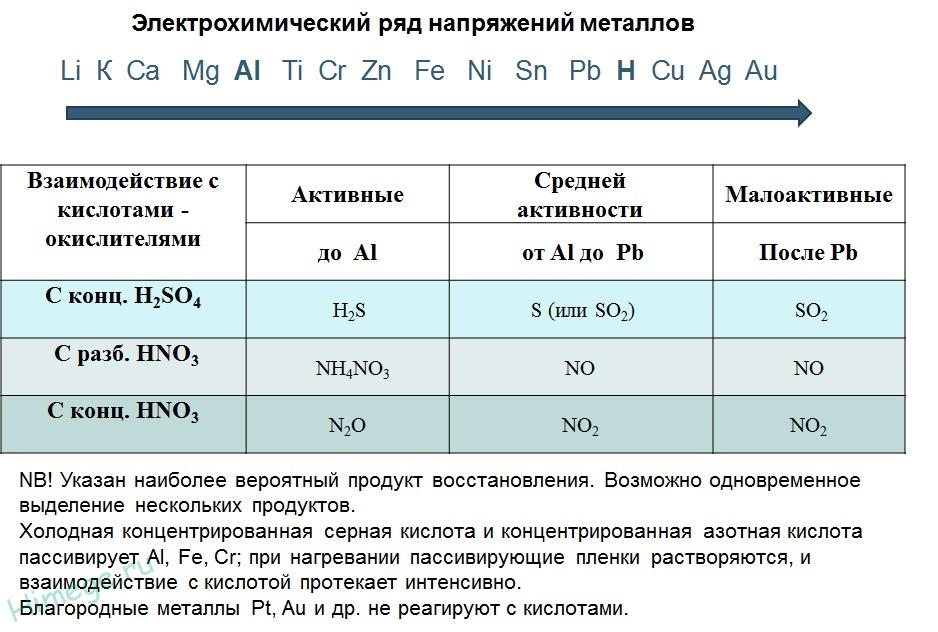
Zn + 2H 2 SO 4 (K) → ZnSO 4 + SO 2 + 2H 2 O
4Zn + 5H 2 SO 4(K) → 4ZnSO 4 + H 2 S + 4H 2 O
3Zn + 4H 2 SO 4(K) → 3ZnSO 4 + S + 4H 2 O
2H 2 SO 4 (c) + Cu → Cu SO 4 + SO 2 + 2H 2 O
10HNO 3 + 4Mg → 4Mg(NO 3) 2 + NH 4 NO 3 + 3H 2 O
4HNO 3 (c) + Сu → Сu (NO 3) 2 + 2NO 2 + 2H 2 O
3. Interaction of metals with water
1) Active (alkali and alkaline earth metals) form a soluble base (alkali) and hydrogen:
2Na + 2H 2 O → 2NaOH + H 2
Ca+ 2H 2 O → Ca(OH) 2 + H 2
2) Metals of medium activity are oxidized by water when heated to oxide:
Zn + H 2 O – t° → ZnO + H 2
3) Inactive (Au, Ag, Pt) - do not react.
4. Displacement by more active metals of less active metals from solutions of their salts:
Cu + HgCl 2 → Hg + CuCl 2
Fe+ CuSO 4 → Cu+ FeSO 4
In industry, not pure metals are often used, but their mixtures - alloys in which the beneficial properties of one metal are complemented by the beneficial properties of another. So, copper has a low hardness and is of little use for the manufacture of machine parts, while alloys of copper with zinc ( brass) are already quite hard and are widely used in mechanical engineering. Aluminum has high ductility and sufficient lightness (low density), but is too soft. On its basis, an alloy with magnesium, copper and manganese is prepared - duralumin (duralumin), which, without losing the useful properties of aluminum, acquires high hardness and becomes suitable in the aircraft industry. Alloys of iron with carbon (and additives of other metals) are widely known cast iron and steel.
Metals in free form are reducing agents. However, the reactivity of some metals is low due to the fact that they are covered with surface oxide film, to varying degrees resistant to the action of such chemical reagents as water, solutions of acids and alkalis.
For example, lead is always covered with an oxide film; its transition into solution requires not only exposure to a reagent (for example, dilute nitric acid), but also heating. The oxide film on aluminum prevents its reaction with water, but is destroyed under the action of acids and alkalis. Loose oxide film (rust), formed on the surface of iron in moist air, does not interfere with the further oxidation of iron.
Under the influence concentrated acids are formed on metals sustainable oxide film. This phenomenon is called passivation. So, in concentrated sulfuric acid passivated (and after that do not react with acid) such metals as Be, Bi, Co, Fe, Mg and Nb, and in concentrated nitric acid - metals A1, Be, Bi, Co, Cr, Fe, Nb, Ni, Pb , Th and U.
When interacting with oxidizing agents in acidic solutions, most metals turn into cations, the charge of which is determined by the stable oxidation state of a given element in compounds (Na +, Ca 2+, A1 3+, Fe 2+ and Fe 3+)
The reducing activity of metals in an acidic solution is transmitted by a series of stresses. Most metals are converted into a solution of hydrochloric and dilute sulfuric acids, but Cu, Ag and Hg - only sulfuric (concentrated) and nitric acids, and Pt and Au - "aqua regia".
Corrosion of metals
An undesirable chemical property of metals is their corrosion, i.e. active destruction (oxidation) upon contact with water and under the influence of oxygen dissolved in it (oxygen corrosion). For example, the corrosion of iron products in water is widely known, as a result of which rust is formed, and the products crumble into powder.
Corrosion of metals proceeds in water also due to the presence of dissolved CO 2 and SO 2 gases; an acidic environment is created, and H + cations are displaced by active metals in the form of hydrogen H 2 ( hydrogen corrosion).
The point of contact between two dissimilar metals can be especially corrosive ( contact corrosion). Between one metal, such as Fe, and another metal, such as Sn or Cu, placed in water, a galvanic couple occurs. The flow of electrons goes from the more active metal, which is to the left in the series of voltages (Re), to the less active metal (Sn, Cu), and the more active metal is destroyed (corrodes).
It is because of this that the tinned surface of cans (tin-plated iron) rusts when stored in a humid atmosphere and carelessly handled (iron quickly collapses after even a small scratch appears, allowing contact of iron with moisture). On the contrary, the galvanized surface of an iron bucket does not rust for a long time, because even if there are scratches, it is not iron that corrodes, but zinc (a more active metal than iron).
Corrosion resistance for a given metal is enhanced when it is coated with a more active metal or when they are fused; for example, coating iron with chromium or making an alloy of iron with chromium eliminates the corrosion of iron. Chrome-plated iron and steel containing chromium ( stainless steel) have high corrosion resistance.