Classification of chemical reactions in inorganic and organic chemistry
The classification of chemical reactions in inorganic and organic chemistry is carried out on the basis of various classifying features, details of which are given in the table below.
By changing the oxidation state of elements
The first sign of classification is by changing the degree of oxidation of the elements that form the reactants and products.
a) redox
b) without changing the oxidation state
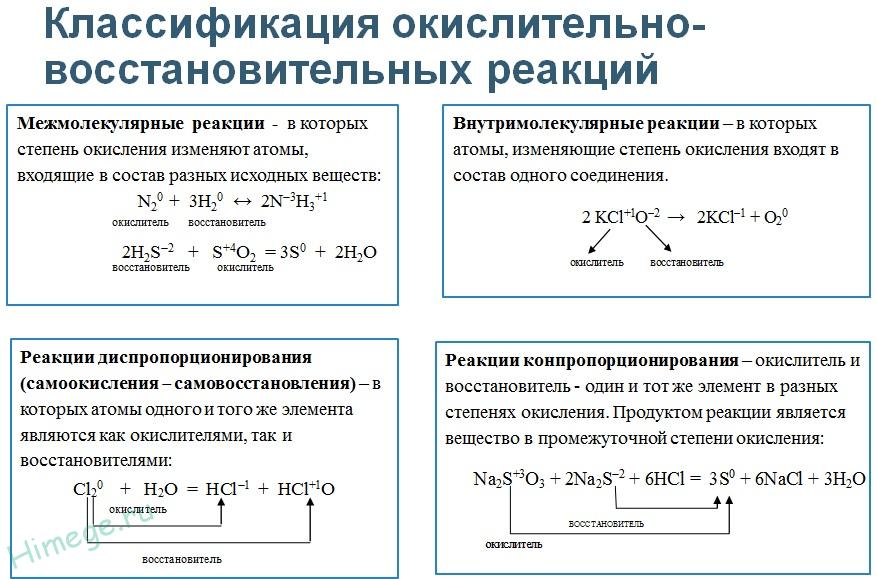
redox called reactions accompanied by a change in the oxidation states of the chemical elements that make up the reagents. Redox in inorganic chemistry includes all substitution reactions and those decomposition and compound reactions in which at least one simple substance is involved. Reactions that proceed without changing the oxidation states of the elements that form the reactants and reaction products include all exchange reactions.
According to the number and composition of reagents and products
Chemical reactions are classified according to the nature of the process, i.e., according to the number and composition of reactants and products.
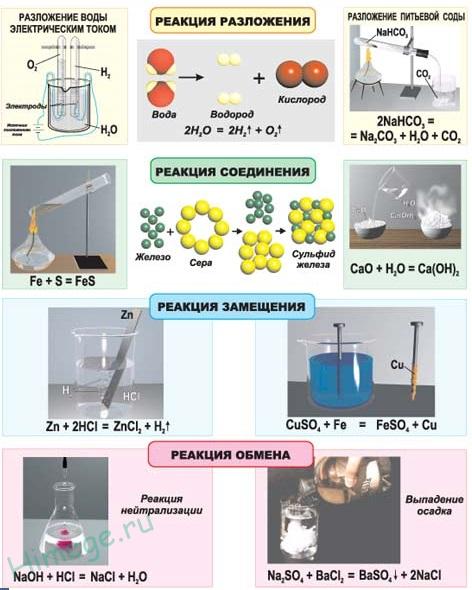
Connection reactions called chemical reactions, as a result of which complex molecules are obtained from several simpler ones, for example:
4Li + O 2 = 2Li 2 O
Decomposition reactions called chemical reactions, as a result of which simple molecules are obtained from more complex ones, for example:
CaCO 3 \u003d CaO + CO 2
Decomposition reactions can be viewed as processes inverse to compound.
substitution reactions chemical reactions are called, as a result of which an atom or group of atoms in a molecule of a substance is replaced by another atom or group of atoms, for example:
Fe + 2HCl \u003d FeCl 2 + H 2
Their distinguishing feature is the interaction of a simple substance with a complex one. Such reactions exist in organic chemistry.
However, the concept of "substitution" in organics is broader than in inorganic chemistry. If in the molecule of the original substance any atom or functional group is replaced by another atom or group, these are also substitution reactions, although from the point of view of inorganic chemistry, the process looks like an exchange reaction.
- exchange (including neutralization).
Exchange reactions call chemical reactions that occur without changing the oxidation states of the elements and lead to the exchange of the constituent parts of the reagents, for example:
AgNO 3 + KBr = AgBr + KNO 3
Run in the opposite direction if possible.
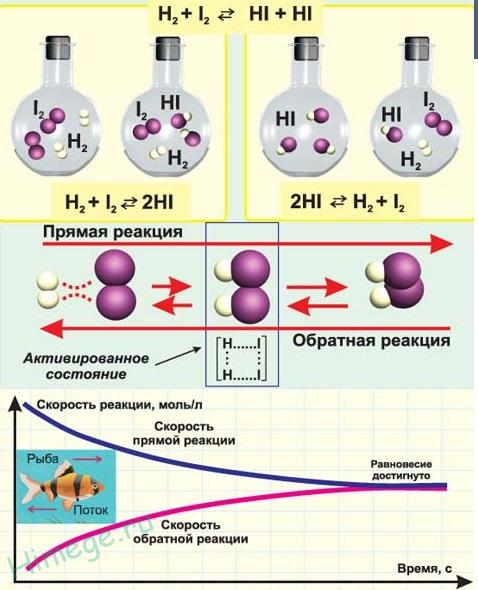
If possible, proceed in the opposite direction - reversible and irreversible.
reversible called chemical reactions occurring at a given temperature simultaneously in two opposite directions with commensurate speeds. When writing the equations of such reactions, the equal sign is replaced by oppositely directed arrows. The simplest example of a reversible reaction is the synthesis of ammonia by the interaction of nitrogen and hydrogen:
N 2 + 3H 2 ↔2NH 3
irreversible are reactions that proceed only in the forward direction, as a result of which products are formed that do not interact with each other. Irreversible include chemical reactions that result in the formation of slightly dissociated compounds, a large amount of energy is released, as well as those in which the final products leave the reaction sphere in gaseous form or in the form of a precipitate, for example:
HCl + NaOH = NaCl + H2O
2Ca + O 2 \u003d 2CaO
BaBr 2 + Na 2 SO 4 = BaSO 4 ↓ + 2NaBr
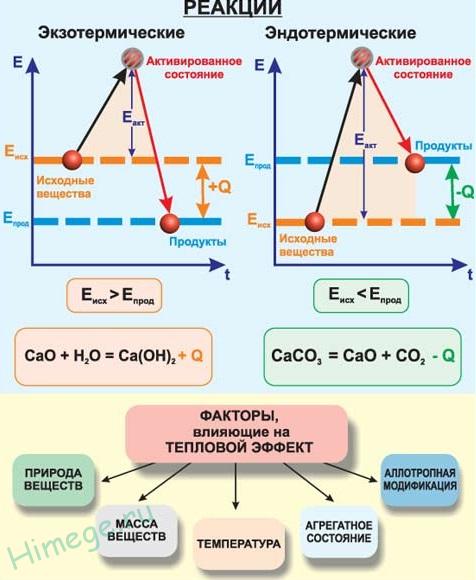
By thermal effect
exothermic are chemical reactions that release heat. The symbol for the change in enthalpy (heat content) is ΔH, and the thermal effect of the reaction is Q. For exothermic reactions, Q > 0, and ΔH< 0.
endothermic called chemical reactions that take place with the absorption of heat. For endothermic reactions Q< 0, а ΔH > 0.
Coupling reactions will generally be exothermic reactions, and decomposition reactions will be endothermic. A rare exception is the reaction of nitrogen with oxygen - endothermic:
N2 + O2 → 2NO - Q
By phase
homogeneous called reactions occurring in a homogeneous medium (homogeneous substances, in one phase, for example, g-g, reactions in solutions).
heterogeneous called reactions that occur in an inhomogeneous medium, on the contact surface of the reacting substances that are in different phases, for example, solid and gaseous, liquid and gaseous, in two immiscible liquids.
By using a catalyst
A catalyst is a substance that speeds up a chemical reaction.
catalytic reactions proceed only in the presence of a catalyst (including enzymatic ones).
Non-catalytic reactions run in the absence of a catalyst.
By type of rupture
According to the type of chemical bond breaking in the initial molecule, homolytic and heterolytic reactions are distinguished.
homolytic called reactions in which, as a result of breaking bonds, particles are formed that have an unpaired electron - free radicals.
Heterolytic called reactions that proceed through the formation of ionic particles - cations and anions.
- homolytic (equal gap, each atom receives 1 electron)
- heterolytic (unequal gap - one gets a pair of electrons)
Radical(chain) chemical reactions involving radicals are called, for example:
CH 4 + Cl 2 hv → CH 3 Cl + HCl
Ionic called chemical reactions that take place with the participation of ions, for example:
KCl + AgNO 3 \u003d KNO 3 + AgCl ↓
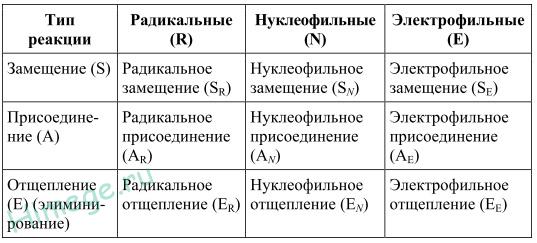
Electrophilic refers to heterolytic reactions of organic compounds with electrophiles - particles that carry a whole or fractional positive charge. They are divided into reactions of electrophilic substitution and electrophilic addition, for example:
C 6 H 6 + Cl 2 FeCl3 → C 6 H 5 Cl + HCl
H 2 C \u003d CH 2 + Br 2 → BrCH 2 -CH 2 Br
Nucleophilic refers to heterolytic reactions of organic compounds with nucleophiles - particles that carry an integer or fractional negative charge. They are subdivided into nucleophilic substitution and nucleophilic addition reactions, for example:
CH 3 Br + NaOH → CH 3 OH + NaBr
CH 3 C (O) H + C 2 H 5 OH → CH 3 CH (OC 2 H 5) 2 + H 2 O
Classification of organic reactions
The classification of organic reactions is given in the table: