1.4.1 Classification of chemical reactions in inorganic and organic chemistry
Lecture: Classification of chemical reactions in inorganic and organic chemistry
Types of chemical reactions in inorganic chemistry
A) Classification by the number of initial substances:
Decomposition - as a result of this reaction, from one existing complex substance, two or more simple, as well as complex substances are formed.
Example: 2H 2 O 2 → 2H 2 O + O 2Compound - this is such a reaction in which two or more simple, as well as complex substances, form one, but more complex.
Example: 4Al+3O 2 → 2Al 2 O 3
substitution - This is a certain chemical reaction that takes place between some simple, as well as complex substances. The atoms of a simple substance, in this reaction, are replaced by atoms of one of the elements found in a complex substance.Example: 2КI + Cl2 → 2КCl + I 2
Exchange - this is such a reaction in which two substances of complex structure exchange their parts.Example: HCl + KNO 2 → KCl + HNO 2
B) Classification by thermal effect:
exothermic reactions - These are certain chemical reactions in which heat is released.Examples:
S + O 2 → SO 2 + Q
2C 2 H 6 + 7O 2 → 4CO 2 + 6H 2 O + Q
Endothermic reactions
are certain chemical reactions in which heat is absorbed. As a rule, these are decomposition reactions.
Examples:
CaCO 3 → CaO + CO 2 - Q
2KClO 3 → 2KCl + 3O 2 - Q
The heat released or absorbed in a chemical reaction is called thermal effect.
Chemical equations in which the heat effect of a reaction is indicated are called thermochemical.
C) Classification by reversibility:
Reversible reactions are reactions that proceed under the same conditions in mutually opposite directions.Example: 3H 2 + N 2 ⇌ 2NH 3
irreversible reactions - these are reactions that proceed only in one direction, as well as culminating in the complete consumption of all starting materials. In these reactions, isolate gas, sediment, water.Example: 2KClO 3 → 2KCl + 3O 2
D) Classification according to the change in the degree of oxidation:
Redox reactions - in the course of these reactions, a change in the degree of oxidation occurs.Example: Сu + 4HNO 3 → Cu(NO 3) 2 + 2NO 2 + 2H 2 O.
Not redox - reactions without changing the oxidation state.Example: HNO 3 + KOH → KNO 3 + H 2 O.
E) Phase classification:
Homogeneous reactions – reactions occurring in one phase, when the starting materials and reaction products have the same state of aggregation.Example: H 2 (gas) + Cl 2 (gas) → 2HCL
heterogeneous reactions - reactions occurring at the phase interface, in which the reaction products and the starting materials have a different state of aggregation.Example: CuO+ H 2 → Cu+H 2 O
Classification by catalyst use:
A catalyst is a substance that speeds up a reaction. A catalytic reaction proceeds in the presence of a catalyst, a non-catalytic reaction without a catalyst.
Example: 2H 2 0 2 MnO2 →
2H 2 O + O 2 catalyst MnO 2
The interaction of alkali with acid proceeds without a catalyst.
Example: KOH + HCl →
KCl + H 2 O
Inhibitors are substances that slow down a reaction.
Catalysts and inhibitors themselves are not consumed during the reaction.
Types of chemical reactions in organic chemistry
substitution - this is a reaction during which one atom / group of atoms is replaced in the original molecule with other atoms / groups of atoms.
Example: CH 4 + Cl 2 → CH 3 Cl + Hcl
Accession are reactions in which several molecules of a substance combine into one. Addition reactions include:
- Hydrogenation is a reaction in which hydrogen is added to a multiple bond.
Example: CH 3 -CH \u003d CH 2 (propene) + H 2 → CH 3 -CH 2 -CH 3 (propane)
Hydrohalogenation is a reaction that adds a hydrogen halide.
Example: CH 2 \u003d CH 2 (ethene) + Hcl → CH 3 -CH 2 -Cl (chloroethane)
Alkynes react with hydrogen halides (hydrogen chloride, hydrogen bromide) in the same way as alkenes. Attachment in a chemical reaction takes place in 2 stages, and is determined by the Markovnikov rule:
When protic acids and water are added to unsymmetrical alkenes and alkynes, a hydrogen atom is attached to the most hydrogenated carbon atom.
The mechanism of this chemical reaction. Formed in the 1st, fast stage, the p-complex in the 2nd slow stage gradually turns into an s-complex - a carbocation. In the 3rd stage, the stabilization of the carbocation occurs - that is, the interaction with the bromine anion:
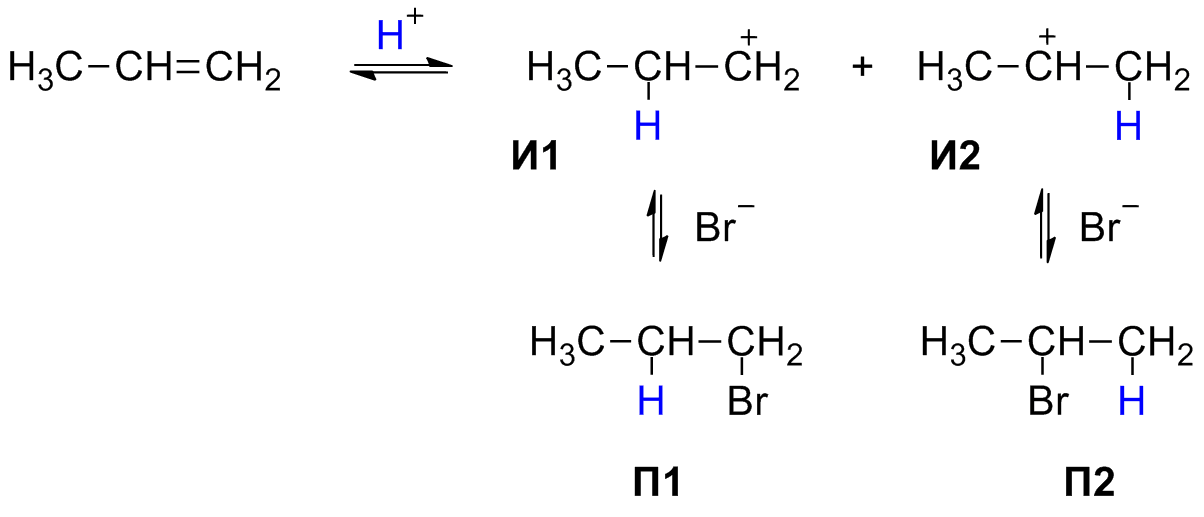
I1, I2 - carbocations. P1, P2 - bromides.
Halogenation A reaction in which a halogen is added. Halogenation is also called all processes, as a result of which halogen atoms are introduced into organic compounds. This concept is used in a "broad sense". In accordance with this concept, the following chemical reactions based on halogenation are distinguished: fluorination, chlorination, bromination, iodination.
Halogen-containing organic derivatives are considered the most important compounds that are used both in organic synthesis and as target products. Halogen derivatives of hydrocarbons are considered to be the starting products in a large number of nucleophilic substitution reactions. As for the practical use of compounds containing halogen, they are used in the form of solvents, such as chlorine-containing compounds, refrigerants - chlorofluoro derivatives, freons, pesticides, pharmaceuticals, plasticizers, monomers for plastics.
Hydration– addition reactions of a water molecule to a multiple bond.
Polymerization - this is a special type of reaction in which molecules of a substance having a relatively small molecular weight join each other, subsequently forming molecules of a substance with a high molecular weight.
| | |